Abstract
Autism spectrum disorders (ASD) are characterized by social and repetitive abnormalities. Although the ASD mouse model with Shank3b mutations is widely used in ASD research, the behavioral phenotype of this model has not been fully elucidated. Here, a 3D-motion capture system and linear discriminant analysis were used to comprehensively record and analyze the behavioral patterns of male and female Shank3b mutant mice. It was found that both sexes replicated the core and accompanied symptoms of ASD, with significant sex differences. Further, Shank3b heterozygous knockout mice exhibited distinct autistic behaviors, that were significantly different from those those observed in the wild type and homozygous knockout groups. Our findings provide evidence for the inclusion of both sexes and experimental approaches to efficiently characterize heterozygous transgenic models, which are more clinically relevant in autistic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorders (ASD) are a diverse group of conditions characterized by atypical patterns of activities, such as repetitive behaviors, motor dysfunction, anxiety, and some degree of difficulty in social interaction and communication [1, 2]. Recent studies have shown that the prevalence of ASD is increasing, estimated 1 in 44 individuals in the United States. ASD affects males more frequently than females, with a male-to-female ratio of 4:1 [3,4,5,6]. Clinically, heterozygous mutations account for most cases underlying ASD patients [7]. The etiology of ASD has been reported to be associated with various genes [8,9,10,11,12], including Shank3 [13,14,15]. Plays an important role in the excitatory synapse, whose disruption or deletion leads to autistic-like behaviors [16,17,18]. Shank3b homozygous knockout (KO) mouse is often used as an ASD model due to its self-injurious grooming, abnormal social interactions, and anxiety-like behaviors [16, 19,20,21,22,23]. Additionally, Shank3 mutations account for approximately 1% of human ASD cases [7]. Therefore, the study of Shank3 mutant mice can provide valuable insights into various aspects of ASD in humans, including behaviors, neural mechanisms, clinical diagnosis, and treatments.
Spontaneous behavior is an essential indicator for evaluating the basic state of animals. 3D capture systems, which combine video tracking systems and machine learning methods, are more efficiently and comprehensively assessing animals’ behavioral phenotypes [24,25,26,27,28,29] and have been used to analyze the spontaneous behavior of animal models with ASD [30, 31]. For example, Huang et al. observed that just a small proportion of female Shank3b KO mice exhibited a weaker tendency for autistic-like behavior than males. Moreover, their study found no significant difference between male and female KO mice at the group level in terms of spontaneous behavior [30]. Another study revealed that male KO mice display more severe impairments than females in motor coordination [32]. However, there is still a lack of adequate evaluation of spontaneous behavior, especially those involving heterozygous and female mice, which could potentially affect our understanding of animals’ natural state due to insufficient observation in new environments.
In this study, by using a 3D-motion capture system, we analyzed the spontaneous behaviors of both male and female Shank3b wild-type littermates (WT), heterozygous (HE), and KO mice, and observed a broader range of behavioral characteristics, including 13 movements and 5 clusters, of Shank3b mutant mice. More detailly, Shank3b KO and HE mice for both sexes exhibited significantly different spatiotemporal behavioral dynamics compared to WT littermates, with the more prominent of the KO groups. For example, both the HE and KO mice spent significantly less time on walking, stepping, and locomotion, but more time on pausing. As to the movements closely related to ASD, such as grooming and hunching, the WT, HE, and KO groups of both sexes exhibited a gradually increased trend. Based on these characteristics, we can easily discriminate the three groups from each other with line discriminant analysis (LDA). Additionally, significant sex differences existed between the male and female mice within the same genotype. Our studies, including both sexes and three genotypes, not only provide comprehensive behavioral data and empirical support for both sexes in experiments utilizing Shank3b mice but also potentially lead to more accurate diagnosis and specific development of effective therapeutic strategies for ASD.
Material and Methods
Animals
Adult Shank3b heterozygous knockout mice (B6.129-Shank3tm2Gfng/J) were purchased from the Jackson Laboratory (Stock No: 017688). To generate wild-type littermates (WT; Shank3b+/+), heterozygotes (HE; Shank3b+/-), and homozygous knockout (KO; Shank3b-/-) mice, 8 to 12-week-old heterozygous males were crossed with age-matched heterozygous females. All mice were housed in cages by genotype, with 3–5 mice per cage, at 20–25°C in a relative humidity of 45%–75%. They were maintained on a 12 h light/dark cycle (lights off at 20:00) and had ad libitum access to standard food and water. Behavioral experiments were performed on mice aged between 10–12 weeks. All husbandry and experimental procedures were approved by Animal Care and Use Committees at the Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences (CAS) (IACUC number: SIAT-IACUC-20210226-ZKYSZXJJSYJY-RZC-WF-A0923-03). To ensure unbiased analysis, all behavioral experiments were performed and analyzed with experimenters blinded to genotypes. The genotypes of transgenic mice were validated by PCR using Primer mix #1 (to distinguish Mut and WT) and Primer mix #2 (to distinguish KO and HE): The Primer mix #1 forward primer was GAGACTGATCAGCGCAGTTG, and the reverse primer was GCTATACGAAGTTATGTCGAC TAGG. The Primer mix #2 forward primer was GAGACTGATCAGCGCAGTTG, and the reverse primer was TGACATAATCGCTGGCAAAG.
Spontaneous Behavioral Test
A spontaneous behavioral test was conducted on heterozygous, homozygous Shank3b mutant mice, and wild-type littermates of both sexes. Mice were gently introduced into a fixed corner in an open field arena and allowed to explore freely in the box for 60 min. The behavior of each mouse was recorded and analyzed. After each test, the chamber was cleaned with 25% ethanol.
The 3D-motion Capture System
The 3D-motion capture system was used to capture the movements of Shank3b mutant mice during the spontaneous behavioral test. The open-field box is made up of a transparent acrylic wall that stands 30 cm tall and a white plastic square floor with sides measuring 40 cm in length. Although a small cuboid (15 cm in length, 18 cm in width, and 15 cm in height) was present in one corner of the open field box, an acrylic transparent partition was placed at the junction of the two boxes to prevent the mouse from accessing the small cuboid free. The open field arena was positioned at the center of a movable stainless-steel support framework measuring 130 × 130 × 90 cm3. The framework had four Intel RealSense D435 cameras mounted orthogonally on its four supporting pillars, and a 56-inch screen was placed horizontally but face down on the top of the shelf to provide uniform and stable white background light. Animals’ behavioral data were extracted from 16 key body parts, including the nose, left ear, right ear, neck, left front limb, right front limb, left hind limb, right hind limb, left front claw, right front claw, left hind claw, right hind claw, back, root tail, middle tail, and tip tail. These key points were used to reconstruct 3D skeletons. The detailed methods, including camera calibration, and animals’ behavioral image acquisition, of this 3D multi-view motion-capture system setup (BA-DC01, Shenzhen Bayone BioTech Co., LTD, Shenzhen), were described in our previous work [30, 33].
Behavior Atlas Analysis
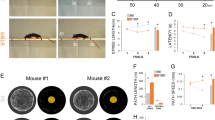
Complex mammalian behavior typically involves both locomotion and non-locomotion movements. Locomotion can be easily identified using velocity-based parameters, whereas non-locomotory movement, which is controlled by many degrees of freedom, is associated with the movement of limbs or organs rather than the torso. In this study, Behavior Atlas was used to analyze the behavioral data acquired by the 3D-motion capture system. The dynamic time alignment kernel was then used to measure similarity among all non-locomotory movement segments and separate them by different features. Uniform manifold approximation and projection (UMAP) was used to reduce the high-dimensional non-locomotory movement features to 2D ones, and 40 behavioral phenotypes were classified by unsupervised clustering. To identify 40 behavior phenotypes more precisely, we manually checked 50 video segments from each cluster center in the feature space. Behavioral movement was identified when the consistency rate of that phenotype cluster was over 80%. The feature space in Fig. 1B demonstrated good-quality clustering of 13 behavioral movements.
Movement identification of Shank3b mutant mice in both sexes. A Experimental paradigm for the multi-view video capture system-guided spontaneous behavioral phenotyping in Shank3b mutant mice of both sexes. B Spatiotemporal feature space of behavioral components. Each dot on the 3D scatter plot represents a movement bout (n = 650 bouts), with the thirteen different colors indicating the corresponding thirteen movements. C Landscape of thirteen movements, with the average skeleton of all frames within each movement phenotype shown from both the top and the side view.
Supervised Movement Annotation
To identify different types of movement phenotypes in Shank3b mutant mice, 100 video clips of each type of movement were randomly selected and manually examined. Similar movements were grouped based on their biological relevance, resulting in the identification of 13 basic types of spontaneous movements. For instance, grooming on different parts of the body (such as the left side, right side, and face) was categorized under a single label grooming. These movements were then further classified into 5 clusters based on their similar behavioral characteristics or specific movements associated with ASD, as detailed in Table 1.
Movements Label Correction
To improve the accuracy of movement recognition, some movement labels were validated against specific rules. For example, the movement sniffing with a horizontal velocity of fewer than 15 mm/s and lasting more than 3 seconds was revised to ‘pausing’.
Discrimination Analysis Across Shank3b WT, HE, and KO Mice
LDA is a classical statistical machine-learning method for supervised classification tasks. To discriminate Shank3b WT littermates, HE, and KO for both sexes, we used time fractions and frequencies of 13 movements, as well as grooming, total distance traveled in the open field arena, time spent in the traditional center and perimeter area, and characteristics of clusters, respectively by LDA.
Temporal Variation of 13 Movements and/or Clusters
To investigate the temporal variation of each movement, we identified every movement and calculated the time fractions and frequencies of each specific movement and/or clusters in every minute with the formula \( T_{i} = \frac{{M_{i} }}{{M_{Total} }}, i \in \left[ {1,60} \right]\). The comprehensive percentage of each minute of movement and/or cluster was calculated by averaging the values of all mice in the same group.
Cluster Transition Probability
For cluster transition probability analysis, each cluster was considered as a state in the probabilistic graphical model [29]. Each cluster fragment, regardless of its interval, was treated as an event and the probabilities of states were calculated based on the number of cluster events. State transition probabilities were calculated based on the frequency of the previous cluster event transformed to the next cluster event. To evaluate the differences in transition probabilities between different groups, we calculated the average differences in transition probability from cluster one to another. Those with a statistically significant P < 0.05 transition probability were excluded (Fig.6E, F, I, J).
Area Division
To accurately determine the boundary between the center and perimeter area in the open field arena, we used the residence time points of the mouse’s back based on the animal’s actual trajectory [29]. We established a consistent coordinate system with the central point of the box as the origin (0,0), and the four corner points calibrated to (−200, 200), (−200, 200), (200, 200), and (−200, −200). Then, we defined a square frame with a side length labeled as ‘a’ that expanded incrementally from the center to the perimeter, to count the residence time points of the mouse’s back within the arena. The growth curve corresponded with the varied residence time points of the mouse's back in the enlarging square frame (Fig. 4A, bottom). The boundary lines were defined by the square frame that lay within 10% of the growth curve's peak prominence. This data-driven method provided enhanced precision in dividing the center and peripheral area based on the animal’s actual trajectory.
Statistical Analysis
All comparisons between groups were made using littermate animals with experiments performed at the same time. The behavioral experiments and data analyses were conducted double-blind. The data were analyzed using Prism 9 (GraphPad 9.0) and presented as means ± SEM, as noted in the figure legends. For the comparison of 13 different movement types and 5 different clusters among WT, HE, and KO mice for both sexes in the spontaneous behavioral test, two-way analysis of variance (ANOVA) was used, Turkey multiple comparisons test was applied as a post hoc analysis. To analyze the time percentage in the different areas among WT, HE, and KO mice of both sexes, we used two-way ANOVA followed by the Dunnett post hoc multiple comparisons test for the analysis of area preference. Statistical analyses of the comparison of different movements and clusters between male and female mice were determined by two-way ANOVA followed by the Turkey post hoc multiple comparisons test. One-way ANOVA was used in the statistical analyses of the total distance traveled in the open field box of both sexes. Statistical significance was denoted by ***P < 0.001, **P < 0.01, *P < 0.05.
Results
Movements Identification by a 3D-Motion Learning Framework
To assess the spontaneous behavior patterns and characteristics of Shank3b mutant mice, we employed a 3D-motion capture system and Behavioral Atlas software [7] (Fig. 1A) to collect and analyze behavioral data from 114 Shank3b mutant mice aged between 12–16 weeks (male: KO = 20, HE = 15, and WT = 19; female: KO = 21, HE = 12, and WT = 17) from both sexes. To accurately capture the motion of different parts of the body, we tracked 16 key body parts to reconstruct the skeleton of a mouse. Using unsupervised clustering, 40 types of behavioral movements were identified. Then, we manually designated 13 types of major behavioral movements based on highly similar postures, including running, trotting, walking, right turning, left turning, stepping, rearing, climbing, hunching, rising, sniffing, grooming, and pausing. Behavioral atlas analysis effectively revealed the structure of the spontaneous behavior in Shank3b mutant mice (Fig. 1B, C) (Table 1).
Different Genotypes Exhibit Different Spontaneous Behavioral Characteristics in Both Sexes
To analyze the spontaneous behavioral repertoires of Shank3b WT, HE, and KO mice, we examined the behavioral sequences (Fig. 2A, Fig. S1A, B) and analyzed time fractions (Fig. 2B) and frequencies (Fig. 2C) of all the movements in the three groups for both sexes over 60 min. Our findings revealed that walking and stepping, which may represent motor deficit function in patients, showed significant differences across the three groups for both sexes (Fig. 2C), and KO mice of both sexes exhibited a significant reduction in other movements such as trotting, right turning, climbing, rising, and sniffing, which may represent the exploratory function in patients, but an increase in grooming, which may represent the stereotyped movements in patients, compared to their WT counterparts. Female KO mice also showed significantly reduced running and increased hunching, which may represent forced posture in patients, compared to the WT group. Interestingly, HE mice from both sexes showed a significant decrease in walking, stepping, and sniffing compared to the KO mice, coupled with a pronounced increase in pausing compared to WT mice and in grooming compared to female KO mice. Furthermore, we evaluated sex differences in all movements across the three groups and found that the frequency of the walking exhibited significant differences between sexes within each genotype (Fig. 2D, Fig. S1C). Specifically, the WT groups displayed the most pronounced differences, followed by the HE group, while the KO group revealed no noticeable differences.
Distinct spontaneous behavioral characteristics of Shank3b mutant mice in both sexes over 60 min. A Representative ethogram of the five significant different movements among Shank3b WT, HE, and KO mice of both male (left) and female (right) mice (Up, WT; Middle, HE; Down, KO). Five animals were randomly selected in each group. Middle color-coded images correspond to the five different movements, top to bottom: walking (orange), stepping (light pink), hunching (blue), grooming (rose red), and pausing (gray). B Comparison of the fraction of thirteen movements among Shank3b WT, HE, and KO mice (Left: male; KO, deep green; HE, pale green; WT, blank; Right: female; KO, dark red; HE, pink; WT, blank). Middle color-coded labels indicate the movements. Statistics: two-way ANOVA followed by Turkey post hoc multiple comparisons test. C Comparison of the frequency of thirteen movements among Shank3b WT, HE, and KO mice (Left: male; Right: female). Statistics: two-way ANOVA followed by Turkey post hoc multiple comparisons test. D Comparison of the frequency of thirteen movements between male and female Shank3b mutant mice (left: WT; Middle: HE; Right: KO). Statistics: two-way ANOVA followed by Turkey post hoc multiple comparisons test. ***P < 0.001, **P < 0.01, *P < 0.05.
Besides, the total travel distance in the open field arena showed that HE and KO mice of both sexes exhibited a significant decrease in walking compared to the WT group (Fig. S2), with the KO group decreasing most prominent. No significant differences were observed between male and female mice of the same genotype group (Fig. S2E–G). Overall, our findings indicated that different genotypes of Shank3b mutant mice exhibited diverse movement characteristics across both sexes.
Genotypes Show Distinct Temporal Patterns of Spontaneous Behaviors
To better understand the functional behavior characteristics of Shank3b WT, HE, and KO mice, we classified 13 movements into 5 clusters based on their similarities or specific links to ASD, including locomotion, exploration, maintenance, forced posture, and nap. Similarly, we analyzed the time fractions and frequencies of each cluster across both sexes over 60 min. Our results showed that time-frequency among all three groups for both sexes showed significant differences in locomotion, with the KO group of the most prominent, mainly attributed to walking and stepping (Fig. 3A, B, I). Further, KO mice exhibited a significant decrease in exploration of both sexes and prominent increases in maintenance and nap for the female group, probably resulting from the increased climbing, grooming, pausing, and decreased sniffing (Fig. 3A, B, I). Additionally, sex differences in the movement fractions (Fig. S3) and frequencies across time (Fig. 3C) of each cluster were examined across the three genotypes. We found that locomotion and exploration of both sexes exhibited significant differences among the three groups. Subsequently, we divided the observational 60-min period into six 10-min segments and conducted an in-depth analysis of the temporal patterns of each cluster (Fig. 3D-H) and movement (Fig. 3I) for both sexes (Fig. S3, S4). Our results showed that both male and female KO mice allocated less time for locomotion cluster across all 10-minute segments, particularly during the last three segments for male HE mice compared with the WT (Fig. 3D). Female KO mice spent significantly more time in the maintenance cluster from the third segment onwards but were only limited to the second segment for the male KO group (Fig. 3E). The notable increase in time spent on the nap cluster of female KO and male HE groups in the whole 60-min period was largely attributed to the significant increase during the last two segments for females and the fourth and final segments for males, respectively (Fig. 3H). No noticeable differences in exploration and forced posture were found in each segment except between the second to fourth segments for the female KO group (Fig. 3F, G). Besides, we conducted a time-based comparison of sex differences in the time spent on the 5 clusters (Fig. S5) and 13 movements between male and female Shank3b WT (Fig. S6), HE (Fig. S7), and KO (Fig. S8) mice. We found that throughout the entire duration, as well as across all six 10-minute segments, there were virtually no significant sex differences observed, indicating a mild sex-dependent variation in the temporal behavioral characteristics among the three groups. These findings provide further insights into different temporal behavioral characteristics among the three groups of Shank3b mutant mice of both sexes.
Temporal dynamics of clusters exhibited by both male and female Shank3b mutant mice. A–B Comparison of the time fractions and frequency of five clusters among male (A left: fractions; Right: frequency) and female (B left: fractions; Right: frequency) Shank3b WT, HE, and KO mice over 60 min. Statistics: two-way ANOVA followed by Turkey post hoc multiple comparisons test. All data are presented as means ± SEM. C Comparison of the frequency of thirteen movements between male and female Shank3b mutant mice (left: WT; Middle: HE; Right: KO). Statistics: two-way ANOVA followed by Turkey post hoc multiple comparisons test. ***P < 0.001, **P < 0.01, *P < 0.05. D–H Temporal dynamics of the five clusters in male and female Shank3b mutant mice over 60 min, with the sequence from D to H representing locomotion, maintenance, forced posture, exploration, and nap. The upper part of the panels shows the results for male mice (KO, deep green; HE, pale green; WT, blank), while the lower part shows the results for female mice (KO, dark red; HE, pink; WT, blank). Statistics: two-way ANOVA followed by Dunnett post hoc multiple comparisons test. All data are presented as means ± SEM. I Temporal dynamics of the 13 movements in every 10-min interval of male (left) and female (right) Shank3b mutant mice. Statistics: two-way ANOVA followed by Dunnett post hoc multiple comparisons test. ***P < 0.001, **P < 0.01, *P < 0.05.
Additionally, we explored the temporal characteristics of the distance traveled by the six groups. Although there was a significant increase in the total distance traveled across all groups and both sexes across all segments, we found a significant decline at each segment for the KO groups and during the last two segments for the HE groups compared to the WT groups (Fig. S9A–D). Notably, the distance traveled in each segment was significantly decreased over time among the three groups, with the most drastic decline being in the KO groups across all segments, while only the fourth and fifth for the male HE groups and the third to fifth segments for female HE groups compared with the WT group, respectively (Fig. S9E–H).
Spatial Preferences in the Open Field Vary Among Different Genotypes
The spatial preferences in the open field test are commonly used to evaluate the anxiety-like behavior in rodents. Typically, the center area is defined as half the length of the open field, and decreased time spent in the center is considered as an increase in anxiety-like level, as shown in Shank3b KO male mice [34, 35]. With the new division method, we found that the length of the square boundaries (‘a’, 136.24 mm, 136.98 mm, and 134.94 mm for WT, HE, and KO mice, respectively) did not differ significantly among WT, HE, and KO mice (Fig. 4A, B). Therefore, we standardized the length at 135 mm for subsequent analysis. The time spent in different areas and types of movements therein were evaluated for Shank3b mutant mice using both traditional and data-driven region division methods. Comparative analyses of two divisions showed that the KO mice of both sexes spent less time in the central area but more time in the perimeter area compared to the WT group (Fig. 4C–H). The same analysis revealed that male KO mice had decreased walking in the centre and decreased pausing in the perimeter area, while female KO mice had decreased walking, stepping, and right turning in the centre and increased hunching, grooming, and pausing in the perimeter area based on the new division. However, only grooming movement significantly increased in the perimeter area in male KO mice, but no noticeable movement significantly decreased in the center area by the traditional division. These findings revealed that our data-driven method is more compatible with the animal's actual trajectory than the traditional ones, suggesting our region division method provides a more detailed insight into behaviors.
Spatial preference characteristics of movements exhibited by male and female Shank3b mutant mice. A Density distribution curve with back coordinates of three Shank3b mutant mice, the red triangle denoting the starting point of the 90% peak prominence as a division of the heterogeneity occupancy density regions. B Representative images of the spatial preference of three Shank3b mutant mice, with the dotted lines delineating the boundary between the center and the perimeter. C, F Comparison of the time fractions in the center and the perimeter among male (C) and female (F) Shank3b WT, HE, and KO mice in 60 min. D, G Comparison of the time fractions per unit area in the center and the perimeter among male (D) and female (G) Shank3b WT, HE, and KO mice in 60 min. E, H The pie diagrams compare the time percentage of the center and perimeter area for male (E) and female (H) Shank3b WT, HE, and KO mice. I Comparison of movement variation in the center (left) and perimeter (right) areas for male Shank3b WT, HE, and KO Mice Over a 60-min duration. J Comparison of movement variations in the center (left) and perimeter (right) areas for female Shank3b WT, HE, and KO mice over a 60-min duration. ***P < 0.001, **P < 0.01, *P < 0.05.
Different Genotypes Display Distinct Behavioral Transition Patterns
Next, we investigated the influence of the genotypes and sexes on the behavioral temporal sequence of Shank3b mice, we analyzed the transition patterns within clusters over an hour. The results showed that the most significant changes and differences among the three groups in both sexes in transition patterns were primarily observed during the first half (Fig. 5A, B). We observed a gradual increase in mutual transition between maintenance and nap across the groups from WT to HE to KO, of both sexes, while the opposite trends were observed between maintenance and locomotion, as well as between forced posture and locomotion (Fig. 5C, D). Moreover, to elucidate the significance of clusters in a behavioral transition network, we computed two-time segments. Our data showed that, during the initial time segment, clusters for both sexes functioned as transitional hubs, manifesting distinct transition patterns when compared to those observed in the WT group. In addition in the HE group, forced posture and nap in males, along with maintenance in females similarly displayed divergent transition patterns relative to WT mice. For example, both the male and female KO mice could transition from locomotion to exploration, as well as to hunching, while locomotion will only transform to exploration in WT mice (Fig. 5E, F). Consistent with the transition patterns of each cluster in the initial time segment, the transition pattern for each cluster as a transitional hub was less pronounced than that in the latter 30-min period (Fig. 5G–J). Overall, mice exhibited relatively stable behavioral connections and transitional patterns, yet transition strategies and behavioral organization of KO and HE groups varied from the WT group.
Behavioral transition probabilities of five clusters in male and female Shank3b mutant mice. A, B Behavioral similarities between the first and the second 30-min duration for male (A) and female (B) Shank3b mutant mice (Left: WT; Middle, HE; Right: KO), with each cell represented the overall similarity of each group within the corresponding timeframes. C, D, G, H Behavioral transition probabilities during the first and the second 30 min across the five clusters in male (first: C; second: G) and female (first: D; second: H) Shank3b WT (left), HE (middle), and KO (right) mice. The five color-coded circles denote the five corresponding clusters. The size of each circle indicates the relative occurrence probability of each cluster, with larger circles denoting higher probabilities. The connecting lines among every two circles represent the transition probabilities of the two clusters, with greater line thickness indicating a higher transition rate. Lines around the circles indicate the self-transition probability based on the movements grouped into the clusters. E, F, I, J Connection properties of each cluster in betweenness centrality for both male (first: E; second: I) and female (first: F; second: J) Shank3b WT, HE, and KO mice during the second 30 min. The magnitude of transition probabilities is symbolized by the length and/or thickness of the lines linking two nodes, with greater length and/or thickness denoting higher probabilities. All lines representing transition probabilities of less than 0.05 are intentionally omitted.
Characterization Data Models Distinguish among Different Genotypes
We explored the feasibility of distinguishing autism-associated mice from WT mice based on the altered spontaneous behavioral patterns exhibited by Shank3b WT, HE, and KO mice. LDA was employed to integrate and analyze the behavioral data of all 13 movements. As expected, Shank3b mutant mice were largely separated from distinct genotypes for both sexes (Fig. 6A, E). We then trained several other LDA classifiers on different parameters that were traditionally applied to discriminate autism, such as grooming, traveling distance, position in the arena, and clusters, to predict genotypes of different groups (Fig. 6B, D, F, H). Our results revealed that prediction accuracy of the classifier based on movement fractions and frequency (discrimination: male, 1.0; female, 0.92) outperformed the classification based on grooming (discrimination: male, 0.48; female, 0.72), distance (discrimination: male, 0.59; female, 0.56), position in the open field arena (discrimination: male, 0.57; female, 0.57), and clusters (discrimination: male, 0.68; female, 0.68) in identifying autistic from WT animals (Fig. 6C, G). This highlights that the classifier trained on 13 movements exhibited more critical insights compared with other behavioral parameters used in most previous methods.
Behavioral dissimilarity among Shank3b WT, HE, and WT across both sexes. A, E Low-dimensional representation of the thirteen movements in male (A) (WT, blank; HE, pale green; KO, deep green) and female (E) (WT, blank; HE, pink; KO, dark red) Shank3b mutant mice using LDA linear model. B, F Normalized classification matrices by all thirteen movements (across rows and columns) for distinguishing male (B) and female (F) Shank3b WT, HE, and KO mice. The numerical values within the matrices represent the count of true and/or predicted instances for each group. The color bar is shared by all matrices. The values, ranging from 0 (depicted in white) to 1 (depicted in black) indicate increasing discrimination. An ideal classifier performance corresponds to a diagonal black with otherwise white fields (Discrimination of 1). C, G The discrimination of different classifiers for male (C) and female (G) Shank3b WT, HE, and KO mice. D, H Normalized classification matrices by traditional classifiers for distinguishing male (D) and female (H) Shank3b WT, HE, and KO mice.
Discussion
In this study, we utilized a 3D-motion capture system to investigate the dynamic characteristics of spontaneous behaviors in Shank3b mutant mice of both sexes. Our results showed that behavioral signatures were significantly different from each other within the same sex among the three groups but more prominent in KO, and across sexes within the same genotype. Further, these three genotypes can be easily discriminated from each other with the LDA approach for both sexes. By including the female mice and HE mice in both sexes, our study provides more comprehensive behavioral data of Shank3b mutant mice, thereby potentially enhancing the efficiency of translational research in ASD.
Although homozygous mouse studies are important for understanding the physiological role of a gene, most human Shank3b patients with clinical conditions are heterozygous mutations. This situation present a great challenge historically to separate from the traditionally developed individuals [36,37,38,39,40]. In this study, By integrating the 3D-motion capture system and LDA algorithm, it is feasible to distinguish HE mice from the WT and KO mice for both sexes (Fig. 6). The reasons were probably due to this advanced system being able to capture a broader spectrum of movements [29, 30], as well as the application of LDA to integrate these movement differences among the three groups. More detailly, ASD patients, including rodents with Shank3 mutations [14, 15, 40], are frequently reported with motor dysfunction [15, 41,42,43]. In our study, we found that compared with the WT littermates, the HE mice demonstrated a significant increase in pausing but a marked decrease in walking and stepping on movement level, as well as locomotion on cluster level, across both sexes. Given the predominance of mutations in clinical ASD cases, identifying HE mice from WT and KO mice allows a more subtle investigation into the pathogenesis with a animal model more relevant to ASD patients, which enhances the potential for developing more precise and effective therapeutic interventions for ASD.
Most previous studies on patients [44] or mouse models of ASD [45, 46] have usually excluded females, probably due to the sex disparity in prevalence. In our study, we examined equal numbers of both male and female mutations and found significant sex differences across the three groups. Specifically, female HE mice showed a significant increase in walking and locomotion than male HE mice, but the difference was smaller than that between the WT groups, while no significant difference between the KO groups (Fig. 2, 3). Similarly, significant sex differences in exploration between male and female mice were observed in both the HE and KO groups, but not from the WT groups (Fig. 3). Our findings showed that different sexes have different behavioral phonotypes in autistic models, suggesting that females should be included in future mechanistic studies and interventional development.
Given the current clinical reliance on behavioral symptoms for ASD diagnoses, acquiring more comprehensive and accurate behavioral phenotypes is of great importance. For example, repetitive behavior deficits, as one of the core symptoms of autism [14,15,16, 47,48,49], are frequently reported in ASD patients, including those with Shank3 mutations [14, 15, 40]. The self-grooming activity of rodents has been widely employed as an index for repetitive behavior. Our 3D method exhibits significant advantages over traditional 2D approaches to the analysis of grooming behavior. In particular our method not only was able to quantify the fractions and frequencies of grooming, but also provide the spatial information of this movement, highlighting a benefit for accurate evaluation of autism-like behaviors. Moreover, as spectrum disorders, no single model can fully mimic the complexity of the human Shank3-related behavioral phenotypes. Hence, incorporating the analysis of social behavior and other different Shank3-related mutations with the 3D-motion capture system will help to establish a more robust behavioral dataset. Additionally, further optimizations in algorithms and improvements in spatiotemporal resolution of movements with this system will significantly enhance our insights into Shank3-associated ASD animals and other disease models. In conclusion, a more detailed understanding of genotype-phenotype correlation in ASD animal models across both sexes will shed light on the neuropathological mechanism of Shank3-associated ASD patients.
References
Battle DE. Diagnostic and statistical manual of mental disorders (DSM). CoDAS 2013, 25: 191–192.
Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet 2019, 51: 431–444.
Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ 2021, 70: 1–16.
Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res 2009, 65: 591–598.
Russell G, Steer C, Golding J. Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Soc Psychiatry Psychiatr Epidemiol 2011, 46: 1283–1293.
Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2017, 56: 466–474.
Leblond CS, Nava C, Polge A, Gauthier J, Huguet G, Lumbroso S, et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A gradient of severity in cognitive impairments. PLoS Genet 2014, 10: e1004580.
Folstein S, Rutter M. Infantile autism: A genetic study of 21 twin pairs. J Child Psychol Psychiatry 1977, 18: 297–321.
Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, et al. Most genetic risk for autism resides with common variation. Nat Genet 2014, 46: 881–885.
Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol Med 1995, 25: 63–77.
Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, et al. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry 2015, 72: 415–423.
Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: A meta-analysis of twin studies. J Child Psychol Psychiatry 2016, 57: 585–595.
Bourgeron T. The possible interplay of synaptic and clock genes in autism spectrum disorders. Cold Spring Harb Symp Quant Biol 2007, 72: 645–654.
Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, et al. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet 2007, 81: 1289–1297.
Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 2007, 39: 25–27.
Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472: 437–442.
Song TJ, Lan XY, Wei MP, Zhai FJ, Boeckers TM, Wang JN, et al. Altered behaviors and impaired synaptic function in a novel rat model with a complete Shank3 deletion. Front Cell Neurosci 2019, 13: 111.
Zhou Y, Sharma J, Ke Q, Landman R, Yuan J, Chen H, et al. Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature 2019, 570: 326–331.
Balasco L, Pagani M, Pangrazzi L, Chelini G, Ciancone Chama AG, Shlosman E, et al. Abnormal whisker-dependent behaviors and altered cortico-hippocampal connectivity in Shank3b-/ - mice. Cereb Cortex 2022, 32: 3042–3056.
Peixoto RT, Chantranupong L, Hakim R, Levasseur J, Wang W, Merchant T, et al. Abnormal striatal development underlies the early onset of behavioral deficits in Shank3B-/- mice. Cell Rep 2019, 29: 2016-2027.e4.
Cope EC, Wang SH, Waters RC, Gore IR, Vasquez B, Laham BJ, et al. Activation of the CA2-ventral CA1 pathway reverses social discrimination dysfunction in Shank3B knockout mice. Nat Commun 2023, 14: 1750.
Peixoto RT, Wang W, Croney DM, Kozorovitskiy Y, Sabatini BL. Early hyperactivity and precocious maturation of corticostriatal circuits in Shank3B (-/-) mice. Nat Neurosci 2016, 19: 716–724.
Chen Q, Deister CA, Gao X, Guo B, Lynn-Jones T, Chen N, et al. Dysfunction of cortical GABAergic neurons leads to sensory hyper-reactivity in a Shank3 mouse model of ASD. Nat Neurosci 2020, 23: 520–532.
Levy DR, Hunter N, Lin S, Robinson EM, Gillis W, Conlin EB, et al. Mouse spontaneous behavior reflects individual variation rather than estrous state. Curr Biol 2023, 33: 1358-1364.e4.
Sheppard K, Gardin J, Sabnis GS, Peer A, Darrell M, Deats S, et al. Stride-level analysis of mouse open field behavior using deep-learning-based pose estimation. Cell Rep 2022, 38: 110231.
Han S, Taralova E, Dupre C, Yuste R. Comprehensive machine learning analysis of Hydra behavior reveals a stable basal behavioral repertoire. Elife 2018, 7: e32605.
Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, et al. Mapping sub-second structure in mouse behavior. Neuron 2015, 88: 1121–1135.
Han Y, Huang K, Chen K, Pan H, Ju F, Long Y, et al. MouseVenue3D: A markerless three-dimension behavioral tracking system for matching two-photon brain imaging in free-moving mice. Neurosci Bull 2022, 38: 303–317.
Ye J, Xu Y, Huang K, Wang X, Wang L, Wang F. Elaborate spontaneous activity atlas-driven behavior patterns in both sexes. 2023, Available at SSRN: https://ssrn.com/abstract=4585835 or https://doi.org/10.2139/ssrn.4585835.
Huang K, Han Y, Chen K, Pan H, Zhao G, Yi W, et al. A hierarchical 3D-motion learning framework for animal spontaneous behavior mapping. Nat Commun 2021, 12: 2784.
de Chaumont F, Ey E, Torquet N, Lagache T, Dallongeville S, Imbert A, et al. Real-time analysis of the behaviour of groups of mice via a depth-sensing camera and machine learning. Nat Biomed Eng 2019, 3: 930–942.
Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet 2011, 20: 3093–3108.
Liu X, Feng X, Huang H, Huang K, Xu Y, Ye S, et al. Male and female mice display consistent lifelong ability to address potential life-threatening cues using different post-threat coping strategies. BMC Biol 2022, 20: 281.
Dhamne SC, Silverman JL, Super CE, Lammers SHT, Hameed MQ, Modi ME, et al. Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism. Mol Autism 2017, 8: 26.
Liu H, Huang X, Xu J, Mao H, Li Y, Ren K, et al. Dissection of the relationship between anxiety and stereotyped self-grooming using the Shank3B mutant autistic model, acute stress model and chronic pain model. Neurobiol Stress 2021, 15: 100417.
Speed HE, Kouser M, Xuan Z, Reimers JM, Ochoa CF, Gupta N, et al. Autism-associated insertion mutation (InsG) of Shank3 exon 21 causes impaired synaptic transmission and behavioral deficits. J Neurosci 2015, 35: 9648–9665.
Jaramillo TC, Speed HE, Xuan Z, Reimers JM, Liu S, Powell CM. Altered striatal synaptic function and abnormal behaviour in Shank3 Exon4-9 deletion mouse model of autism. Autism Res 2016, 9: 350–375.
Kouser M, Speed HE, Dewey CM, Reimers JM, Widman AJ, Gupta N, et al. Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. J Neurosci 2013, 33: 18448–18468.
Drapeau E, Riad M, Kajiwara Y, Buxbaum JD. Behavioral phenotyping of an improved mouse model of phelan-McDermid syndrome with a complete deletion of the Shank3 gene. eNeuro 2018, 5: ENEURO.0046–ENEURO.0018.2018.
Matas E, Maisterrena A, Thabault M, Balado E, Francheteau M, Balbous A, et al. Major motor and gait deficits with sexual dimorphism in a Shank3 mutant mouse model. Mol Autism 2021, 12: 2.
Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. J Autism Dev Disord 2010, 40: 1227–1240.
Yirmiya N, Charman T. The prodrome of autism: Early behavioral and biological signs, regression, peri- and post-natal development and genetics. J Child Psychol Psychiatry 2010, 51: 432–458.
Al Sagheer T, Haida O, Balbous A, Francheteau M, Matas E, Fernagut PO, et al. Motor impairments correlate with social deficits and restricted neuronal loss in an environmental model of autism. Int J Neuropsychopharmacol 2018, 21: 871–882.
Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: An update. J Autism Dev Disord 2003, 33: 365–382.
Wang Q, Kong Y, Wu DY, Liu JH, Jie W, You QL, et al. Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice. Nat Commun 2021, 12: 3321.
Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83: 1131–1143.
Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci 2010, 30: 2115–2129.
Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 2010, 35: 976–989.
Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 2010, 11: 490–502.
Acknowledgments
This work was supported by the National Science and Technology Innovation 2030-Major Project of China (2021ZD0201003 and 2022ZD0208300); the National Natural Science Foundation of China (32171092); the Shenzhen Key Basic Research Project (JCYJ20220818100805013); Shenzhen Key Laboratory of Viral Vectors for Biomedicine (ZDSYS202008111424010 05); Guangdong Provincial Medical Products Administration Key Laboratory of Quality Control Technology for Virus-Based Therapeutics (2020ZDB26); Guangdong Provincial Medical Products Administration (2022ZDZ13). We would like to express our sincere gratitude to the members of Feng Wang’s lab for their helpful comments and kind suggestions regarding the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors claim that there are no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, J., Ye, J., Ji, C. et al. Mapping the Behavioral Signatures of Shank3b Mice in Both Sexes. Neurosci. Bull. (2024). https://doi.org/10.1007/s12264-024-01237-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12264-024-01237-8