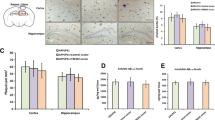
Abstract
Deficiencies in the clearance of peripheral amyloid β (Aβ) play a crucial role in the progression of Alzheimer’s disease (AD). Previous studies have shown that the ability of blood monocytes to phagocytose Aβ is decreased in AD. However, the exact mechanism of Aβ clearance dysfunction in AD monocytes remains unclear. In the present study, we found that blood monocytes in AD mice exhibited decreases in energy metabolism, which was accompanied by cellular senescence, a senescence-associated secretory phenotype, and dysfunctional phagocytosis of Aβ. Improving energy metabolism rejuvenated monocytes and enhanced their ability to phagocytose Aβ in vivo and in vitro. Moreover, enhancing blood monocyte Aβ phagocytosis by improving energy metabolism alleviated brain Aβ deposition and neuroinflammation and eventually improved cognitive function in AD mice. This study reveals a new mechanism of impaired Aβ phagocytosis in monocytes and provides evidence that restoring their energy metabolism may be a novel therapeutic strategy for AD.






Similar content being viewed by others
References
Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5: e661–e671.
Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 2016, 8: 595–608.
Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, et al. The amyloid-β pathway in Alzheimer’s disease. Mol Psychiatry 2021, 26: 5481–5503.
Cao W, Zheng H. Peripheral immune system in aging and Alzheimer’s disease. Mol Neurodegeneration 2018, 13: 51.
Yan P, Kim KW, Xiao Q, Ma X, Czerniewski LR, Liu H, et al. Peripheral monocyte-derived cells counter amyloid plaque pathogenesis in a mouse model of Alzheimer’s disease. J Clin Invest 2022, 132: e152565.
Wu KM, Zhang YR, Huang YY, Dong Q, Tan L, Yu JT. The role of the immune system in Alzheimer’s disease. Ageing Res Rev 2021, 70: 101409.
Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet 2019, 51: 404–413.
Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 2019, 51: 414–430.
Zhang DF, Fan Y, Xu M, Wang G, Wang D, Li J, et al. Complement C7 is a novel risk gene for Alzheimer’s disease in Han Chinese. Natl Sci Rev 2019, 6: 257–274.
Bartels T, De Schepper S, Hong S. Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Science 2020, 370: 66–69.
Xiang Y, Bu XL, Liu YH, Zhu C, Shen LL, Jiao SS, et al. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol 2015, 130: 487–499.
Yuede CM, Lee H, Restivo JL, Davis TA, Hettinger JC, Wallace CE, et al. Rapid in vivo measurement of β-amyloid reveals biphasic clearance kinetics in an Alzheimer’s mouse model. J Exp Med 2016, 213: 677–685.
Chen SH, He CY, Shen YY, Zeng GH, Tian DY, Cheng Y, et al. Polysaccharide Krestin prevents Alzheimer’s disease-type pathology and cognitive deficits by enhancing monocyte amyloid-β processing. Neurosci Bull 2022, 38: 290–302.
Chen SH, Tian DY, Shen YY, Cheng Y, Fan DY, Sun HL, et al. Amyloid-beta uptake by blood monocytes is reduced with ageing and Alzheimer’s disease. Transl Psychiatry 2020, 10: 423.
Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38: 633–643.
Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol 2017, 18: 488–498.
Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat Immunol 2019, 20: 50–63.
Pan RY, Ma J, Kong XX, Wang XF, Li SS, Qi XL, et al. Sodium rutin ameliorates Alzheimer’s disease-like pathology by enhancing microglial amyloid-β clearance. Sci Adv 2019, 5: eaau6328.
Griffiths HR, Gao D, Pararasa C. Redox regulation in metabolic programming and inflammation. Redox Biol 2017, 12: 50–57.
Brandt D. Pence, Aging impairs mitochondrial respiratory capacity in classical monocytes. Exp Gerontol 2018, 108: 112–117.
Saare M, Tserel L, Haljasmägi L, Taalberg E, Peet N, Eimre M, et al. Monocytes present age-related changes in phospholipid concentration and decreased energy metabolism. Aging Cell 2020, 19: e13127.
Minhas PS, Latif-Hernandez A, McReynolds MR, Durairaj AS, Wang Q, Rubin A, et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 2021, 590: 122–128.
Chen ZY, Zhang Y. Animal models of Alzheimer’s disease: Applications, evaluation, and perspectives. Zool Res 2022, 43: 1026–1040.
Yu ZY, Chen DW, Tan CR, Zeng GH, He CY, Wang J, et al. Physiological clearance of Aβ by spleen and splenectomy aggravates Alzheimer-type pathogenesis. Aging Cell 2022, 21: e13533.
Tian DY, Cheng Y, Zhuang ZQ, He CY, Pan QG, Tang MZ, et al. Physiological clearance of amyloid-beta by the kidney and its therapeutic potential for Alzheimer’s disease. Mol Psychiatry 2021, 26: 6074–6082.
Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease—Insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol 2017, 13: 612–623.
Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, et al. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J Alzheimers Dis 2005, 7: 221–232;discussion 255–262.
Hu Y, Mai W, Chen L, Cao K, Zhang B, Zhang Z, et al. mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP. Glia 2020, 68: 1031–1045.
O’Neill LAJ, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med 2016, 213: 15–23.
McIntosh A, Mela V, Harty C, Minogue AM, Costello DA, Kerskens C, et al. Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol 2019, 29: 606–621.
Holland R, McIntosh AL, Finucane OM, Mela V, Rubio-Araiz A, Timmons G, et al. Inflammatory microglia are glycolytic and iron retentive and typify the microglia in APP/PS1 mice. Brain Behav Immun 2018, 68: 183–196.
Pan RY, He L, Zhang J, Liu X, Liao Y, Gao J, et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab 2022, 34: 634-648.e6.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell 2023, 186: 243–278.
Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol 2014, 32: 609–634.
Li Q, Peng J, Luo Y, Zhou J, Li T, Cao L, et al. Far infrared light irradiation enhances Aβ clearance via increased exocytotic microglial ATP and ameliorates cognitive deficit in Alzheimer’s disease-like mice. J Neuroinflammation 2022, 19: 145.
Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021, 594: 100–105.
De Maeyer RPH, Chambers ES. The impact of ageing on monocytes and macrophages. Immunol Lett 2021, 230: 1–10.
Zhang YR, Wang JJ, Chen SF, Wang HF, Li YZ, Ou YN, et al. Peripheral immunity is associated with the risk of incident dementia. Mol Psychiatry 2022, 27: 1956–1962.
Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat Rev Neurol 2021, 17: 157–172.
Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol 2014, 14: 463–477.
Prokop S, Miller KR, Drost N, Handrick S, Mathur V, Luo J, et al. Impact of peripheral myeloid cells on amyloid-β pathology in Alzheimer’s disease-like mice. J Exp Med 2015, 212: 1811–1818.
Varvel NH, Grathwohl SA, Degenhardt K, Resch C, Bosch A, Jucker M, et al. Replacement of brain-resident myeloid cells does not alter cerebral amyloid-β deposition in mouse models of Alzheimer’s disease. J Exp Med 2015, 212: 1803–1809.
Monoranu CM, Hartmann T, Strobel S, Heinsen H, Riederer P, Distel L, et al. Is there any evidence of monocytes involvement in alzheimer’s disease? A pilot study on human postmortem brain. J Alzheimers Dis Rep 2021, 5: 887–897.
Park JC, Baik SH, Han SH, Cho HJ, Choi H, Kim HJ, et al. Annexin A1 restores Aβ1-42-induced blood-brain barrier disruption through the inhibition of RhoA-ROCK signaling pathway. Aging Cell 2017, 16: 149–161.
Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, et al. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing 2015, 12: 2.
Sterling K, Xing M, Song W. Do systemic infections contribute to the pathogenesis of dementia? Neurosci Bull 2022, 38: 331–333.
Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, et al. ABAD directly links Aß to mitochondrial toxicity in Alzheimer’s disease. Science 2004, 304: 448–452.
Parks JK, Smith TS, Trimmer PA, Bennett JP Jr, Davis Parker W, Jr. Neurotoxic Aβ peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J Neurochem 2001, 76: 1050–1056.
Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann MG, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med 2008, 14: 1097–1105.
Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, et al. An uncoupling channel within the c-subunit ring of the F 1 F O ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 2014, 111: 10580–10585.
Peng Y, Gao P, Shi L, Chen L, Liu J, Long J. Central and peripheral metabolic defects contribute to the pathogenesis of Alzheimer’s disease: Targeting mitochondria for diagnosis and prevention. Antioxid Redox Signal 2020, 32: 1188–1236.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82122023 and U22A20294).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, ZH., Bai, YD., Yu, ZY. et al. Improving Blood Monocyte Energy Metabolism Enhances Its Ability to Phagocytose Amyloid-β and Prevents Alzheimer’s Disease-Type Pathology and Cognitive Deficits. Neurosci. Bull. 39, 1775–1788 (2023). https://doi.org/10.1007/s12264-023-01077-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-023-01077-y