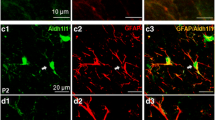
Abstract
Abnormal synchronous neuronal activity has been widely detected by brain imaging of autistic patients, but its underlying neural mechanism remains unclear. Compared with wild-type mice, our in vivo two-photon imaging showed that transgenic (Tg1) mice over-expressing human autism risk gene MeCP2 exhibited higher neuronal synchrony in the young but lower synchrony in the adult stage. Whole-cell recording of neuronal pairs in brain slices revealed that higher neuronal synchrony in young postnatal Tg1 mice was attributed mainly to more prevalent giant slow inward currents (SICs). Both in vivo and slice imaging further demonstrated more dynamic activity and higher synchrony in astrocytes from young Tg1 mice. Blocking astrocytic gap junctions markedly decreased the generation of SICs and overall cell synchrony in the Tg1 brain. Furthermore, the expression level of Cx43 protein and the coupling efficiency of astrocyte gap junctions remained unchanged in Tg1 mice. Thus, astrocytic gap junctions facilitate but do not act as a direct trigger for the abnormal neuronal synchrony in young Tg1 mice, revealing the potential role of the astrocyte network in the pathogenesis of MeCP2 duplication syndrome.






Similar content being viewed by others
References
Christensen DL, Maenner MJ, Bilder D, Constantino JN, Daniels J, Durkin MS. Prevalence and characteristics of autism spectrum disorder among children aged 4 years: early autism and developmental disabilities monitoring network, seven sites, United States, 2010, 2012, and 2014. MMWR Surveill Summ 2019, 68: 1–19.
Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, et al. Prevalence of autism spectrum disorder among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ 2020, 69: 1–12.
Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psych 2011, 168: 904–912.
Zhou H, Xu X, Yan W, Zou X, Wu L, Luo X, et al. Prevalence of autism spectrum disorder in China: a nationwide multi-center population-based study among children aged 6 to 12 years. Neurosci Bull 2020, 36: 961–971.
Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord 2006, 36: 5–25.
Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 2006, 52: 155–168.
Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci 2004, 24: 9228–9231.
Ameis SH, Catani M. Altered white matter connectivity as a neural substrate for social impairment in Autism spectrum disorder. Cortex 2015, 62: 158–181.
Keehn B, Wagner JB, Tager-Flusberg H, Nelson CA. Functional connectivity in the first year of life in infants at-risk for autism: a preliminary near-infrared spectroscopy study. Front Hum Neurosci 2013, 7: 444.
Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psych 2013, 70: 869–879.
Keown CL, Shih P, Nair A, Peterson N, Mulvey ME, Müller RA. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Rep 2013, 5: 567–572.
Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep 2013, 5: 738–747.
Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 2002, 125: 1839–1849.
Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism. Intercorrelations of regional glucose utilization. Arch Neurol 1988, 45: 749–755.
d’Albis MA, Guevara P, Guevara M, Laidi C, Boisgontier J, Sarrazin S, et al. Local structural connectivity is associated with social cognition in autism spectrum disorder. Brain 2018, 141: 3472–3481.
Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol 2005, 15: 225–230.
Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 2007, 17: 103–111.
Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Front Syst Neurosci 2011, 5: 10.
Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev 2012, 36: 1292–1313.
Lu H, Ash RT, He LJ, Kee SE, Wang W, Yu DH, et al. Loss and gain of MeCP2 cause similar hippocampal circuit dysfunction that is rescued by deep brain stimulation in a rett syndrome mouse model. Neuron 2016, 91: 739–747.
Ko H, Hofer SB, Pichler B, Buchanan KA, Sjöström PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature 2011, 473: 87–91.
Renart A, de la Rocha J, Bartho P, Hollender L, Parga N, Reyes A, et al. The asynchronous state in cortical circuits. Science 2010, 327: 587–590.
Maheswaranathan N, Ferrari S, Vandongen AM, Henriquez CS. Emergent bursting and synchrony in computer simulations of neuronal cultures. Front Comput Neurosci 2012, 6: 15.
Ostojic S, Brunel N, Hakim V. How connectivity, background activity, and synaptic properties shape the cross-correlation between spike trains. J Neurosci 2009, 29: 10234–10253.
Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 2008, 60: 430–440.
Wilton DK, Dissing-Olesen L, Stevens B. Neuron-glia signaling in synapse elimination. Annu Rev Neurosci 2019, 42: 107–127.
Nimmerjahn A, Bergles DE. Large-scale recording of astrocyte activity. Curr Opin Neurobiol 2015, 32: 95–106.
Zonta M, Carmignoto G. Calcium oscillations encoding neuron-to-astrocyte communication. J Physiol Paris 2002, 96: 193–198.
Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 2004, 43: 729–743.
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 2005, 57: 67–81.
Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci 2009, 12: 311–317.
Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci 2009, 29: 5051–5061.
Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, et al. A role for glia in the progression of Rett’s syndrome. Nature 2011, 475: 497–500.
Dong QP, Liu Q, Li RH, Wang AX, Bu Q, Wang KH, et al. Mechanism and consequence of abnormal calcium homeostasis in Rett syndrome astrocytes. Elife 2018, 7: e33417.
Wang Q, Kong Y, Wu DY, Liu JH, Jie W, You QL, et al. Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice. Nat Commun 2021, 12: 3321.
Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet 2004, 13: 2679–2689.
Yu B, Yuan B, Dai JK, Cheng TL, Xia SN, He LJ, et al. Reversal of social recognition deficit in adult mice with MECP2 duplication via normalization of MeCP2 in the medial prefrontal cortex. Neurosci Bull 2020, 36: 570–584.
Guizar-Sicairos M, Thurman ST, Fienup JR. Efficient subpixel image registration algorithms. Opt Lett 2008, 33: 156–158.
Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng HK, et al. Ca2+ signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Nat Neurosci 2015, 18: 708–717.
Tong XP, Shigetomi E, Looger LL, Khakh BS. Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist 2013, 19: 274–291.
Leighton AH, Lohmann C. The wiring of developing sensory circuits-from patterned spontaneous activity to synaptic plasticity mechanisms. Front Neural Circuits 2016, 10: 71.
Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci 2010, 13: 1433–1440.
Golomb D. Neuronal synchrony measures. Scholarpedia 2007, 2: 1347.
Golomb D, Shedmi A, Curtu R, Ermentrout GB. Persistent synchronized bursting activity in cortical tissues with low magnesium concentration: a modeling study. J Neurophysiol 2006, 95: 1049–1067.
Tischbirek CH, Noda T, Tohmi M, Birkner A, Nelken I, Konnerth A. In vivo functional mapping of a cortical column at single-neuron resolution. Cell Rep 2019, 27: 1319-1326.e5.
Tada M, Takeuchi A, Hashizume M, Kitamura K, Kano M. A highly sensitive fluorescent indicator dye for calcium imaging of neural activity in vitro and in vivo. Eur J Neurosci 2014, 39: 1720–1728.
Schnell C, Hagos Y, Hülsmann S. Active sulforhodamine 101 uptake into hippocampal astrocytes. PLoS ONE 2012, 7: e49398. https://doi.org/10.1371/journal.pone.0049398.
Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 2004, 1: 31–37.
Gonçalves JT, Anstey JE, Golshani P, Portera-Cailliau C. Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci 2013, 16: 903–909.
Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci 2013, 7: 458.
Doiron B, Litwin-Kumar A, Rosenbaum R, Ocker GK, Josić K. The mechanics of state-dependent neural correlations. Nat Neurosci 2016, 19: 383–393.
Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 2007, 56: 58–65.
Na ES, Nelson ED, Adachi M, Autry AE, Mahgoub MA, Kavalali ET, et al. A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. J Neurosci 2012, 32: 3109–3117.
de la Rocha J, Doiron B, Shea-Brown E, Josić K, Reyes A. Correlation between neural spike trains increases with firing rate. Nature 2007, 448: 802–806.
Chen Q, Deister CA, Gao X, Guo BL, Lynn-Jones T, Chen NY, et al. Dysfunction of cortical GABAergic neurons leads to sensory hyper-reactivity in a Shank3 mouse model of ASD. Nat Neurosci 2020, 23: 520–532.
Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 2006, 86: 1009–1031.
Fellin T, Pascual O, Haydon PG. Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiol (Bethesda) 2006, 21: 208–215.
Pál B. Astrocytic actions on extrasynaptic neuronal currents. Front Cell Neurosci 2015, 9: 474.
Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, et al. Ro 25–6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther 1997, 283: 1285–1292.
Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 2009, 32: 421–431.
Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev 2018, 98: 239–389.
Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 2014, 81: 728–739.
Volterra A, Liaudet N, Savtchouk I. Astrocyte Ca2+ signalling: An unexpected complexity. Nat Rev Neurosci 2014, 15: 327–335.
Bindocci E, Savtchouk I, Liaudet N, Becker D, Carriero G, Volterra A. Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science 2017, 356: eaai8185.
Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci 2001, 4: 803–812.
Kovács A, Pál B. Astrocyte-dependent slow inward currents (SICs) participate in neuromodulatory mechanisms in the pedunculopontine nucleus (PPN). Front Cell Neurosci 2017, 11: 16.
Navarrete M, Perea G, Maglio L, Pastor J, García de Sola R, Araque A. Astrocyte calcium signal and gliotransmission in human brain tissue. Cereb Cortex 2013, 23: 1240–1246.
Mariotti L, Losi G, Sessolo M, Marcon I, Carmignoto G. The inhibitory neurotransmitter GABA evokes long-lasting Ca(2+) oscillations in cortical astrocytes. Glia 2016, 64: 363–373.
Martin PE, Wall C, Griffith TM. Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. Br J Pharmacol 2005, 144: 617–627.
Nagy JI, Yamamoto T, Sawchuk MA, Nance DM, Hertzberg EL. Quantitative immunohistochemical and biochemical correlates of connexin43 localization in rat brain. Glia 1992, 5: 1–9.
Chahrour M, Jung SY, Shaw C, Zhou XB, Wong ST, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320: 1224–1229.
Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron 2007, 56: 422–437.
Jiang MH, Ash RT, Baker SA, Suter B, Ferguson A, Park J, et al. Dendritic arborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. J Neurosci 2013, 33: 19518–19533.
Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci 1998, 18: 3870–3896.
Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 2000, 32: 1–14.
Pirttimaki TM, Sims RE, Saunders G, Antonio SA, Codadu NK, Parri HR. Astrocyte-mediated neuronal synchronization properties revealed by false gliotransmitter release. J Neurosci 2017, 37: 9859–9870.
Yu XZ, Taylor AMW, Nagai NG, Golshani P, Evans CJ, Coppola G, et al. Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 2018, 99: 1170-1187.e9.
Ma ZG, Stork T, Bergles DE, Freeman MR. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 2016, 539: 428–432.
Pannasch U, Vargová L, Reingruber J, Ezan P, Holcman D, Giaume C, et al. Astroglial networks scale synaptic activity and plasticity. Proc Natl Acad Sci U S A 2011, 108: 8467–8472.
Tani E, Nishiura M, Higashi N. Freeze-fracture studies of gap junctions of normal and neoplastic astrocytes. Acta Neuropathol 1973, 26: 127–138.
Buskila Y, Bellot-Saez A, Morley JW. Generating brain waves, the power of astrocytes. Front Neurosci 2019, 13: 1125.
Houades V, Koulakoff A, Ezan P, Seif I, Giaume C. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J Neurosci 2008, 28: 5207–5217.
Acknowledgements
We thank Dr. Zilong Qiu for providing the Tg1 mouse line, and Dr. Yu Xin for assistance with statistical analysis. We are grateful to Prof. Mu-ming Poo and Dr. Zilong Qiu for critical reading of the manuscript. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB32010100), a National Natural Science Foundation of China project (31671113), a Shanghai Municipal Science and Technology Major Project (2018SHZDZX05), and the State Key Laboratory of Neuroscience.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no potential conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xia, S., Xu, HT. Astrocytic Gap Junctions Contribute to Aberrant Neuronal Synchronization in a Mouse Model of MeCP2 Duplication Syndrome. Neurosci. Bull. 38, 591–606 (2022). https://doi.org/10.1007/s12264-022-00824-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-022-00824-x