Abstract
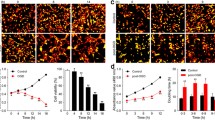
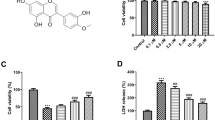
Stroke is an acute cerebro-vascular disease with high incidence and poor prognosis, most commonly ischemic in nature. In recent years, increasing attention has been paid to inflammatory reactions as symptoms of a stroke. However, the role of inflammation in stroke and its underlying mechanisms require exploration. In this study, we evaluated the inflammatory reactions induced by acute ischemia and found that pyroptosis occurred after acute ischemia both in vivo and in vitro, as determined by interleukin-1β, apoptosis-associated speck-like protein, and caspase-1. The early inflammation resulted in irreversible ischemic injury, indicating that it deserves thorough investigation. Meanwhile, acute ischemia decreased the Sirtuin 1 (Sirt1) protein levels, and increased the TRAF6 (TNF receptor associated factor 6) protein and reactive oxygen species (ROS) levels. In further exploration, both Sirt1 suppression and TRAF6 activation were found to contribute to this pyroptosis. Reduced Sirt1 levels were responsible for the production of ROS and increased TRAF6 protein levels after ischemic exposure. Moreover, N-acetyl-L-cysteine, an ROS scavenger, suppressed the TRAF6 accumulation induced by oxygen-glucose deprivation via suppression of ROS bursts. These phenomena indicate that Sirt1 is upstream of ROS, and ROS bursts result in increased TRAF6 levels. Further, the activation of Sirt1 during the period of ischemia reduced ischemia-induced injury after 72 h of reperfusion in mice with middle cerebral artery occlusion. In sum, these results indicate that pyroptosis-dependent machinery contributes to the neural injury during acute ischemia via the Sirt1-ROS-TRAF6 signaling pathway. We propose that inflammatory reactions occur soon after oxidative stress and are detrimental to neuronal survival; this provides a promising therapeutic target against ischemic injuries such as a stroke.








Similar content being viewed by others
References
Katan M, Luft A. Global burden of stroke. Semin Neurol 2018, 38: 208–211.
Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol 1998, 56: 149–171.
Moskowitz MA. Brain protection: maybe yes, maybe no. Stroke 2010, 41: S85–86.
Del Zoppo GJ, Saver JL, Jauch EC, Adams HP, Jr., American Heart Association Stroke C. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke 2009, 40: 2945–2948.
Sekerdag E, Solaroglu I, Gursoy-Ozdemir Y. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr Neuropharmacol 2018, 16: 1396–1415.
Shukla V, Shakya AK, Perez-Pinzon MA, Dave KR. Cerebral ischemic damage in diabetes: an inflammatory perspective. J Neuroinflammation 2017, 14: 21.
Adamczak SE, de Rivero Vaccari JP, Dale G, Brand FJ, 3rd, Nonner D, Bullock MR, et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab 2014, 34: 621–629.
Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. J Neuroinflammation 2010, 7: 74.
Ahmad M, Graham SH. Inflammation after stroke: mechanisms and therapeutic approaches. Transl Stroke Res 2010, 1: 74–84.
Xie P. TRAF molecules in cell signaling and in human diseases. J Mol Signal 2013, 8: 7.
Lee NK, Lee SY. Modulation of life and death by the tumor necrosis factor receptor-associated factors (TRAFs). J Biochem Mol Biol 2002, 35: 61–66.
Lomaga MA, Henderson JT, Elia AJ, Robertson J, Noyce RS, Yeh WC, et al. Tumor necrosis factor receptor-associated factor 6 (TRAF6) deficiency results in exencephaly and is required for apoptosis within the developing CNS. J Neurosci 2000, 20: 7384–7393.
Zhang J, Fu B, Zhang X, Chen L, Zhang L, Zhao X, et al. Neuroprotective effect of bicyclol in rat ischemic stroke: down-regulates TLR4, TLR9, TRAF6, NF-kappaB, MMP-9 and up-regulates claudin-5 expression. Brain Res 2013, 1528: 80–88.
Rajendran R, Garva R, Krstic-Demonacos M, Demonacos C. Sirtuins: molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. J Biomed Biotechnol 2011, 2011: 368276.
Xu J, Jackson CW, Khoury N, Escobar I, Perez-Pinzon MA. Brain SIRT1 mediates metabolic homeostasis and neuroprotection. Front Endocrinol (Lausanne) 2018, 9: 702.
Duan J, Yin Y, Wei G, Cui J, Zhang E, Guan Y, et al. Chikusetsu saponin IVa confers cardioprotection via SIRT1/ERK1/2 and Homer1a pathway. Sci Rep 2015, 5: 18123.
Blokh D, Stambler I. Information theoretical analysis of aging as a risk factor for heart disease. Aging Dis 2015, 6: 196–207.
Meng X, Tan J, Li M, Song S, Miao Y, Zhang Q. Sirt1: Role under the condition of ischemia/hypoxia. Cell Mol Neurobiol 2017, 37: 17–28.
Wang CP, Shi YW, Tang M, Zhang XC, Gu Y, Liang XM, et al. Isoquercetin ameliorates cerebral impairment in focal ischemia through anti-oxidative, anti-inflammatory, and anti-apoptotic effects in primary culture of rat hippocampal neurons and hippocampal CA1 region of rats. Mol Neurobiol 2017, 54: 2126–2142.
Hadj Abdallah N, Baulies A, Bouhlel A, Bejaoui M, Zaouali MA, Ben Mimouna S, et al. Zinc mitigates renal ischemia-reperfusion injury in rats by modulating oxidative stress, endoplasmic reticulum stress, and autophagy. J Cell Physiol 2018, 233: 8677–8690.
Nasoohi S, Ismael S, Ishrat T. Thioredoxin-interacting protein (TXNIP) in cerebrovascular and neurodegenerative diseases: Regulation and implication. Mol Neurobiol 2018, 55: 7900–7920.
Zhang N, Yin Y, Han S, Jiang J, Yang W, Bu X, et al. Hypoxic preconditioning induced neuroprotection against cerebral ischemic injuries and its cPKCgamma-mediated molecular mechanism. Neurochem Int 2011, 58: 684–692.
Wang YP, Wang ZF, Zhang YC, Tian Q, Wang JZ. Effect of amyloid peptides on serum withdrawal-induced cell differentiation and cell viability. Cell Res 2004, 14: 467–472.
Evans MS, Collings MA, Brewer GJ. Electrophysiology of embryonic, adult and aged rat hippocampal neurons in serum-free culture. J Neurosci Methods 1998, 79: 37–46.
Sundar R, Gudey SK, Heldin CH, Landstrom M. TRAF6 promotes TGFbeta-induced invasion and cell-cycle regulation via Lys63-linked polyubiquitination of Lys178 in TGFbeta type I receptor. Cell Cycle 2015, 14: 554–565.
Muller L, Tokay T, Porath K, Kohling R, Kirschstein T. Enhanced NMDA receptor-dependent LTP in the epileptic CA1 area via upregulation of NR2B. Neurobiol Dis 2013, 54: 183–193.
Li Y, Sun W, Han S, Li J, Ding S, Wang W, et al. IGF-1-involved negative feedback of NR2B NMDA subunits protects cultured hippocampal neurons against NMDA-induced excitotoxicity. Mol Neurobiol 2017, 54: 684–696.
Yumnam S, Venkatarame Gowda Saralamma V, Raha S, Lee HJ, Lee WS, Kim EK, et al. Proteomic profiling of human HepG2 cells treated with hesperidin using antibody array. Mol Med Rep 2017, 16: 5386–5392.
Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002, 10: 417–426.
Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 2012, 4.
Abdullah M, Berthiaume JM, Willis MS. Tumor necrosis factor receptor-associated factor 6 as a nuclear factor kappa B-modulating therapeutic target in cardiovascular diseases: at the heart of it all. Transl Res 2018, 195: 48–61.
Ren M, Guo Y, Wei X, Yan S, Qin Y, Zhang X, et al. TREM2 overexpression attenuates neuroinflammation and protects dopaminergic neurons in experimental models of Parkinson’s disease. Exp Neurol 2018, 302: 205–213.
Lee CW, Chi MC, Hsu LF, Yang CM, Hsu TH, Chuang CC, et al. Carbon monoxide releasing molecule-2 protects against particulate matter-induced lung inflammation by inhibiting TLR2 and 4/ROS/NLRP3 inflammasome activation. Mol Immunol 2019, 112: 163–174.
Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci U S A 2002, 99: 16162–16167.
Andrabi SS, Ali M, Tabassum H, Parveen S, Parvez S. Pramipexole prevents ischemic cell death via mitochondrial pathways in ischemic stroke. Dis Model Mech 2019, 12. https://doi.org/10.1242/dmm.033860
Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999, 22: 391–397.
Barrett KM, Lal BK, Meschia JF. Stroke: advances in medical therapy and acute stroke intervention. Curr Cardiol Rep 2015, 17: 79.
del Zoppo GJ. Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci 2010, 1207: 143–148.
Drieu A, Martinez de Lizarrondo S, Rubio M. Stopping inflammation in stroke: Role of ST2/IL-33 signaling. J Neurosci 2017, 37: 9614–9616.
Kalaivani P, Ganesh M, Sathiya S, Ranju V, Gayathiri V, Saravana Babu C. Alteration in bioenergetic regulators, SirT1 and Parp1 expression precedes oxidative stress in rats subjected to transient cerebral focal ischemia: molecular and histopathologic evidences. J Stroke Cerebrovasc Dis 2014, 23: 2753–2766.
Petegnief V, Planas AM. SIRT1 regulation modulates stroke outcome. Transl Stroke Res 2013, 4: 663–671.
Lv H, Wang L, Shen J, Hao S, Ming A, Wang X, et al. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res Bull 2015, 115: 30–36.
Liang X, Liu Y, Jia S, Xu X, Dong M, Wei Y. SIRT1: The value of functional outcome, stroke-related dementia, anxiety, and depression in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2019, 28: 205–212.
Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang YJ, et al. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med 2009, 47: 229–240.
Settembre C, Annunziata I, Spampanato C, Zarcone D, Cobellis G, Nusco E, et al. Systemic inflammation and neurodegeneration in a mouse model of multiple sulfatase deficiency. Proc Natl Acad Sci U S A 2007, 104: 4506–4511.
Chen J, Wu X, Shao B, Zhao W, Shi W, Zhang S, et al. Increased expression of TNF receptor-associated factor 6 after rat traumatic brain injury. Cell Mol Neurobiol 2011, 31: 269–275.
Ten VS, Starkov A. Hypoxic-ischemic injury in the developing brain: the role of reactive oxygen species originating in mitochondria. Neurol Res Int 2012, 2012: 542976.
Hu S, Cheng D, Peng D, Tan J, Huang Y, Chen C. Leptin attenuates cerebral ischemic injury in rats by modulating the mitochondrial electron transport chain via the mitochondrial STAT3 pathway. Brain Behav 2019, 9: e01200.
Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2014, 2: 702–714.
Zhang Y, Liu L, Peng YL, Liu YZ, Wu TY, Shen XL, et al. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci Ther 2014, 20: 119–124.
Touzani O, Boutin H, Chuquet J, Rothwell N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol 1999, 100: 203–215.
Wang X, Yue TL, Barone FC, White RF, Gagnon RC, Feuerstein GZ. Concomitant cortical expression of TNF-alpha and IL-1 beta mRNAs follows early response gene expression in transient focal ischemia. Mol Chem Neuropathol 1994, 23: 103–114.
Brough D, Tyrrell PJ, Allan SM. Regulation of interleukin-1 in acute brain injury. Trends Pharmacol Sci 2011, 32: 617–622.
Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci 2001, 21: 5528–5534.
Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, et al. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol 2011, 187: 4835–4843.
Terlizzi M, Molino A, Colarusso C, Donovan C, Imitazione P, Somma P, et al. Activation of the absent in melanoma 2 inflammasome in peripheral blood mononuclear cells from idiopathic pulmonary fibrosis patients leads to the release of pro-fibrotic mediators. Front Immunol 2018, 9: 670.
Luheshi NM, Kovacs KJ, Lopez-Castejon G, Brough D, Denes A. Interleukin-1alpha expression precedes IL-1beta after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J Neuroinflammation 2011, 8: 186.
Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell 2014, 157: 1013–1022.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 2016, 16: 407–420.
Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 2019, 570: 338–343.
Stancu IC, Cremers N, Vanrusselt H, Couturier J, Vanoosthuyse A, Kessels S, et al. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol 2019, 137: 599–617.
Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014, 156: 1193–1206.
Lu A, Li Y, Schmidt FI, Yin Q, Chen S, Fu TM, et al. Molecular basis of caspase-1 polymerization and its inhibition by a new capping mechanism. Nat Struct Mol Biol 2016, 23: 416–425.
Wang LJ, Colella R, Yorke G, Roisen FJ. The ganglioside GM1 enhances microtubule networks and changes the morphology of Neuro-2a cells in vitro by altering the distribution of MAP2. Exp Neurol 1996, 139: 1–11.
Kamelgarn M, Chen J, Kuang L, Jin H, Kasarskis EJ, Zhu H. ALS mutations of FUS suppress protein translation and disrupt the regulation of nonsense-mediated decay. Proc Natl Acad Sci U S A 2018, 115: E11904–E11913.
Reddy PH, Manczak M, Yin X. Mitochondria-division inhibitor 1 protects against amyloid-beta induced mitochondrial fragmentation and synaptic damage in Alzheimer’s disease. J Alzheimers Dis 2017, 58: 147–162.
LePage KT, Dickey RW, Gerwick WH, Jester EL, Murray TF. On the use of neuro-2a neuroblastoma cells versus intact neurons in primary culture for neurotoxicity studies. Crit Rev Neurobiol 2005, 17: 27–50.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31771292 and 31571162). We thank Professor Heping Cheng, Peking University, for kindly supporting the analytical methods for the fluorescence images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, W., Sun, W., Fan, J. et al. Sirt1-ROS-TRAF6 Signaling-Induced Pyroptosis Contributes to Early Injury in Ischemic Mice. Neurosci. Bull. 36, 845–859 (2020). https://doi.org/10.1007/s12264-020-00489-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-020-00489-4