Abstract
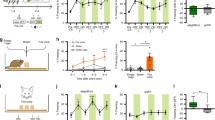
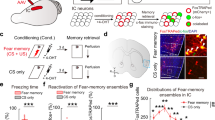
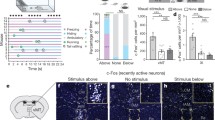
Emotional contagion, a primary form of empathy, is present in rodents. Among emotional contagion behaviors, social transmission of fear is the most studied. Here, we modified a paradigm used in previous studies to more robustly assess the social transmission of fear in rats that experienced foot-shock. We used resting-state functional magnetic resonance imaging to show that foot-shock experience enhances the regional connectivity of the anterior cingulate cortex (ACC). We found that lesioning the ACC specifically attenuated the vicarious freezing behavior of foot-shock-experienced observer rats. Furthermore, ablation of projections from the ACC to the mediodorsal thalamus (MDL) bilaterally delayed the vicarious freezing responses, and activation of these projections decreased the vicarious freezing responses. Overall, our results demonstrate that, in rats, the ACC modulates vicarious freezing behavior via a projection to the MDL and provide clues to understanding the mechanisms underlying empathic behavior in humans.






Similar content being viewed by others
Change history
28 August 2020
The authors found that in this article
References
de Waal FBM, Preston SD. Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci 2017, 18: 498–509.
Frith U, Happe F. Autism spectrum disorder. Curr Biol 2005, 15: R786–790.
Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos Trans R Soc Lond B Biol Sci 2009, 364: 1377–1383.
Achim AM, Ouellet R, Roy MA, Jackson PL. Assessment of empathy in first-episode psychosis and meta-analytic comparison with previous studies in schizophrenia. Psychiatry Res 2011, 190: 3–8.
Horan WP, Reise SP, Kern RS, Lee J, Penn DL, Green MF. Structure and correlates of self-reported empathy in schizophrenia. J Psychiatr Res 2015, 66-67: 60–66.
Derntl B, Finkelmeyer A, Toygar TK, Hulsmann A, Schneider F, Falkenberg DI, et al. Generalized deficit in all core components of empathy in schizophrenia. Schizophrenia Res 2009, 108: 197–206.
de Waal FBM. Putting the altruism back into altruism: The evolution of empathy. Annu Rev Psychol 2008, 59: 279–300.
Zaki J, Ochsner K. The neuroscience of empathy: progress, pitfalls and promise. Nat Neurosci 2012, 15: 675–680.
Decety J. The neuroevolution of empathy. Ann N Y Acad Sci 2011, 1231: 35–45.
Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One 2009, 4: e4387.
Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci 2010, 13: 482–488.
Sivaselvachandran S, Acland EL, Abdallah S, Martin LJ. Behavioral and mechanistic insight into rodent empathy. Neurosci Biobehav Rev 2018, 91: 130–137.
Chen J. Empathy for distress in humans and rodents. Neurosci Bull 2018, 34: 216–236.
Liddle MJ, Bradley BS, McGrath A. Baby empathy: infant distress and peer prosocial responses. Infant Ment Health J 2015, 36: 446–458.
Kim S, Matyas F, Lee S, Acsady L, Shin HS. Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proc Natl Acad Sci U S A 2012, 109: 15497–15501.
Twining RC, Vantrease JE, Love S, Padival M, Rosenkranz JA. An intra-amygdala circuit specifically regulates social fear learning. Nat Neurosci 2017, 20: 459–469.
Guzman YF, Tronson NC, Guedea A, Huh KH, Gao C, Radulovic J. Social modeling of conditioned fear in mice by non–fearful conspecifics. Behav Brain Res 2009, 201: 173–178.
D’Amato FR. Kin interaction enhances morphine analgesia in male mice. Behav Pharmacol 1998, 9: 369–373.
Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang CJ, Felix-Ortiz AC, et al. Corticoamygdala transfer of socially derived information gates observational learning. Cell 2018, 173: 1329-1342 e1318.
Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One 2010, 5: e15077.
Keum S, Kim A, Shin JJ, Kim JH, Park J, Shin HS. A missense variant at the Nrxn3 locus enhances empathy fear in the mouse. Neuron 2018, 98: 588–601.e585.
Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci 2010, 30: 4999–5007.
Kovacs B, Keri S. Off-label intranasal oxytocin use in adults is associated with increased amygdala-cingulate resting-state connectivity. Eur Psychiatry 2015, 30: 542–547.
Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 2011, 12: 524–538.
Pisansky MT, Hanson LR, Gottesman, II, Gewirtz JC. Oxytocin enhances observational fear in mice. Nat Commun 2017, 8: 2102.
Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci 2012, 35: 1–23.
Bolkan SS, Stujenske JM, Parnaudeau S, Spellman TJ, Rauffenbart C, Abbas AI, et al. Thalamic projections sustain prefrontal activity during working memory maintenance. Nat Neurosci 2017, 20: 987–996.
Cardoso-Cruz H, Sousa M, Vieira JB, Lima D, Galhardo V. Prefrontal cortex and mediodorsal thalamus reduced connectivity is associated with spatial working memory impairment in rats with inflammatory pain. Pain 2013, 154: 2397–2406.
Rikhye RV, Gilra A, Halassa MM. Thalamic regulation of switching between cortical representations enables cognitive flexibility. Nat Neurosci 2018, 21: 1753–1763.
Marton TF, Seifikar H, Luongo FJ, Lee AT, Sohal VS. Roles of prefrontal cortex and mediodorsal thalamus in task engagement and behavioral flexibility. J Neurosci 2018, 38: 2569–2578.
Jett JD, Bulin SE, Hatherall LC, McCartney CM, Morilak DA. Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex. Neuroscience 2017, 346: 284–297.
Parnaudeau S, Taylor K, Bolkan SS, Ward RD, Balsam PD, Kellendonk C. Mediodorsal thalamus hypofunction impairs flexible goal-directed behavior. Biol Psychiatry 2015, 77: 445–453.
Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex 2014, 24: 3116–3130.
Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry 2012, 169: 1092–1099.
Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry 2015, 72: 882–891.
Zhou Y, Fan L, Qiu C, Jiang T. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci Bull 2015, 31: 207–219.
Valdes-Hernandez PA, Sumiyoshi A, Nonaka H, Haga R, Aubert-Vasquez E, Ogawa T, et al. An in vivo MRI template set for morphometry, tissue segmentation, and fMRI localization in rats. Front Neuroinform 2011, 5: 26.
Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004, 22: 394–400.
Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 2011, 6: e25031.
Wu T, Grandjean J, Bosshard SC, Rudin M, Reutens D, Jiang T. Altered regional connectivity reflecting effects of different anaesthesia protocols in the mouse brain. Neuroimage 2017, 149: 190–199.
Yin Y, Jin CF, Eyler LT, Jin H, Hu XL, Duan L, et al. Altered regional homogeneity in post-traumatic stress disorder: a restingstate functional magnetic resonance imaging study. Neurosci Bull 2012, 28: 541–549.
Peng CY, Chen YC, Cui Y, Zhao DL, Jiao Y, Tang TY, et al. Regional coherence alterations revealed by resting-state fMRI in post-stroke patients with cognitive dysfunction. PLoS One 2016, 11: e0159574.
Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, et al. Experience modulates vicarious freezing in rats: a model for empathy. PLoS One 2011, 6: e21855.
Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci 2014, 369: 20130146.
Matyas F, Lee J, Shin HS, Acsady L. The fear circuit of the mouse forebrain: connections between the mediodorsal thalamus, frontal cortices and basolateral amygdala. Eur J Neurosci 2014, 39: 1810–1823.
Sanders J, Mayford M, Jeste D. Empathic fear responses in mice are triggered by recognition of a shared experience. PLoS One 2013, 8: e74609.
Padilla-Coreano N, Do-Monte FH, Quirk GJ. A time-dependent role of midline thalamic nuclei in the retrieval of fear memory. Neuropharmacology 2012, 62: 457–463.
Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM. Thalamic amplification of cortical connectivity sustains attentional control. Nature 2017, 545: 219–223.
Alcaraz F, Fresno V, Marchand AR, Kremer EJ, Coutureau E, Wolff M. Thalamocortical and corticothalamic pathways differentially contribute to goal-directed behaviors in the rat. Elife 2018, 7.
Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol 1908, 18: 459–482.
Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 2012, 76: 223–239.
Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994, 116: 143–151.
Liu D, Gu XW, Zhu J, Zhang XX, Han Z, Yan WJ, et al. Medial prefrontal activity during delay period contributes to learning of a working memory task. Science 2014, 346: 458–463.
Barraza JA, Zak PJ. Empathy toward strangers triggers oxytocin release and subsequent generosity. Ann N Y Acad Sci 2009, 1167: 182–189.
Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, et al. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol Psychiatry 2011, 70: 291–297.
Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, et al. A distributed network for social cognition enriched for oxytocin receptors. J Neurosci 2016, 36: 2517–2535.
Li K, Nakajima M, Ibanez-Tallon I, Heintz N. A cortical circuit for sexually dimorphic oxytocin-dependent anxiety behaviors. Cell 2016, 167: 60–72 e11.
Acknowledgements
We thank Dr. Dingcheng Wu for help in data analysis. We are also grateful for technical support from the Optical Imaging Facility and the Animal Facility of the Institute of Neuroscience, Chinese Academy of Sciences. This work was supported in part by The National Key Research and Development Program of China (2017YFB1300200, 2017YFB1300203) and the National Natural Science Foundation of China (61627808).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng, C., Huang, Y., Bo, B. et al. Projection from the Anterior Cingulate Cortex to the Lateral Part of Mediodorsal Thalamus Modulates Vicarious Freezing Behavior. Neurosci. Bull. 36, 217–229 (2020). https://doi.org/10.1007/s12264-019-00427-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-019-00427-z