Abstract
Surgery is a key factor for the curative treatment of hepatoblastoma. Recent evidence suggests that liver transplantation has a strong effect in treating advanced hepatoblastoma. However, there is no consensus on the effects of liver transplantation. This meta-analysis aims to identify the efficacy and safety of liver transplantation for advanced hepatoblastoma, compared with those of conventional liver resection. Electronic databases were searched for relevant studies published prior to June 2022 to evaluate the survival benefit and safety in patients with advanced hepatoblastoma. The primary outcomes were the overall survival and disease-free survival rates, and the secondary outcomes were the complication and tumor recurrence rates. Five relevant clinical studies with a total of 134 participants were included in this meta-analysis. Compared with aggressive liver resection, liver transplantation had similar overall survival rates and disease-free survival rates after 1 year, 3 years, and 5 years (odds ratio (OR) 1 year = 0.89, 95% CI 0.21–3.79, P = 0.88; OR 3 years = 0.54, 95% CI 0.16–1.81, P = 0.32; OR 5 years = 1.24, 95% CI 0.22–6.82, P = 0.81; OR disease-free 1 year = 2.17, 95% CI 0.56–8.42, P = 0.26; OR disease-free 3 years = 1.42, 95% CI 0.48–4.17, P = 0.53; OR disease-free 5 years = 2.91, 95% CI 0.56–8.52, P = 0.26), tumor recurrence rates (OR = 0.62, 95% CI 0.24–1.60, P = 0.32), and complication rates (OR = 1.46, 95% CI 0.48–4.49, P = 0.51). Sensitivity analysis also demonstrated the same outcomes in terms of the tumor recurrence rate, complication rate, and overall and disease-free survival rates after 1 year, 3 years, and 5 years. The funnel plot indicated a low publication bias. Liver transplantation is an excellent option for advanced hepatoblastoma in children with acceptable perioperative complications, which is not inferior to liver resection. And liver transplantation should be considered the primary curative option when liver resection is not possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatoblastoma is the most frequent malignant hepatic tumor in children, with an annual increase of 4.3% in incidence [1, 2]. Between one-third and two-thirds of patients mostly diagnosed under the age of 3 initially present in an advanced stage (irresectable or unresponsive to chemotherapy), but up to 85% of patients can be completely operable after significant downstaging by neoadjuvant chemotherapy [3-5].
Hepatoblastoma generally remains asymptomatic. The diagnosis and staging of hepatoblastoma are identified by α-fetoprotein (AFP), Doppler ultrasound, computed tomography (CT), and magnetic resonance imaging [6]. As per the SIOP protocol, the PRE-Treatment EXTent of disease (PRETEXT) system, a surgical staging system, is used for pretreatment staging of the tumor [7]. A previous study investigated whether AFP levels < 100 ng/ml or > 1.2 × 106 ng/mL, tumor recurrence, PRETEXT IV, metastases, and nonresponse to chemotherapy were related to poor prognosis [8, 9]. Hepatoblastoma is considered advanced if the following conditions are present: multifocal or large solitary PRETEXT III/IV tumor; unifocal centrally located tumor with invasion of all main hilar structures and/or all 3 hepatic veins; PRETEXT II tumor with metastatic involvement; PRETEXT III tumor with involvement of 2 or 3 nonadjacent hepatic segments; or recurrent tumor [10-12].
Thanks to advancements in surgical resection, including conventional liver resection (LR) and liver transplantation (LT), chemotherapy and postoperative care, chemotherapy combined with surgical resection has significantly improved the therapeutic effect on hepatoblastoma in the past 30 years, especially for advanced cases [13-15]. Hepatoblastoma has been proven to be sensitive to chemotherapy, and most patients can achieve complete resection after neoadjuvant chemotherapy [16]. LR, as a first-line radical surgical resection, is still the primary therapy for resectable hepatoblastoma. However, in irresectable cases, especially in infants, aggressive LR after successful tumor downstaging suffers from the challenges of tumor residue and small-for-size syndrome resulting from the excessive loss of hepatic tissue [17, 18]. LT is, in turn, an excellent option for these cases. Some previous studies have suggested that liver transplantation after chemotherapy showed excellent outcomes in terms of the survival rate, tumor recurrence rate, and complications for advanced hepatoblastoma [3, 15, 19-21]. However, this view remains controversial [22, 23]. Consequently, establishing a therapeutic schedule for advanced hepatoblastoma between aggressive LR and alternatively LT may be an extremely difficult task. Moreover, there are currently no systematic reviews or meta-analysis studies about LT in advanced hepatoblastoma in electronic databases. Thus, this meta-analysis aims to identify the efficacy and safety of LT for advanced hepatoblastoma, compared with those of aggressive LR.
Patients and Methods
Information Sources, Search, Study Objectives, Inclusion and Exclusion Criteria, and Extraction
Trials were identified by searching PubMed, Springer Link, China National Knowledge Infrastructure Database (CNKI), VIP Journal Integration Platform (http://www.cqvip.com) (VIP), and WanFang Database (WanFang). All searches included studies established prior to June 2022 using “hepatoblastoma,” AND “liver transplantation,” AND “resection.” Searches were performed for all types of publications but limited to original articles in English or Chinese. We also manually screened the relevant references of the retrieved articles or published clinical trials. The resulting outcomes were divided into two types.
The primary outcomes were the overall survival rate and disease-free survival rate of advanced hepatoblastoma patients treated with LT versus liver resection. The secondary outcomes were the complication rate and tumor recurrence rate.
All published studies evaluating the effects of LT versus LR were included. The treatment group was composed of patients undergoing LT, and the control group was composed of patients undergoing LR. The participants with advanced hepatoblastoma in the selected studies were under 18 years of age. A randomized-controlled clinical trial was the first option, and case series analysis was the second option. Studies were excluded if they: (a) did not meet the above criteria; (b) were repeat studies or had overlapping cases; (c) were systematic reviews or meta-analyses; or (d) had no comparative data for the primary or secondary outcomes.
Two of the authors independently assessed the eligibility of potential studies based on the selection criteria and the extracted data (e.g., outcomes extracted with a data extraction form, author and year of publication, study methods, participants’ characteristics). The concordance rate of the two reviewers was 98%. Disagreements were resolved by consensus.
Quality Assessment and Statistical Analysis
The quality of the retrieved trials was evaluated by two independent reviewers (Jiazhi Li and Dan Wu), according to the randomization method, blinding of tests, allocation concealment, reporting of dropouts in clinical studies, and lack of follow up [24]. Disagreements were discussed, and consensus was reached after discussion. A clinical study was considered of high quality if it reported both the randomization method and allocation concealment in detail, of moderate quality if it only reported either the randomization methods or allocation concealment, and of low quality if it did not report the randomization method or allocation concealment or was a case series analysis [25].
All data for this meta-analysis were analyzed using Review Manager software (RevMan Version 5.2, The Cochrane Collaboration; The Nordic Cochrane Center, Copenhagen, Denmark). Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to estimate the therapeutic effects. The overall effect was considered to be statistically significant if P values were < 0.05. The I2 statistic and associated P values were used to evaluate the heterogeneity among trials [26, 27]. Homogeneity (I2 value < 25%), low heterogeneity (I2 value between 25 and 50%), moderate heterogeneity (I2 value between 50 and 75%), and high heterogeneity (I2 value > 75%) were used to measure the inconsistency across studies. Furthermore, statistically significant heterogeneity was considered to exist in the included studies if P values were < 0.1, and then a random-effects model instead of a fixed-effects model was used to analyze the results. The causes were also explored, including the staging and size of the tumor and differences in interventions and chemotherapy. Potential publication bias was assessed by the symmetry of the funnel plot, and the visual symmetrical plot indicated that the publication bias among studies was low [27].
Results
Identification, Characteristics, and Methodological Quality of the Included Studies
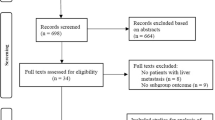
After the initial screening, 598 potentially relevant clinical trials with homogeneity were identified. A total of 567 studies were excluded after screening their titles, including duplicate studies (Fig. 1). The last or the most complete data were extracted for the duplicated studies. After full assessment for eligibility by the two independent reviewers, twenty-six studies were excluded: (a) twenty studies lacked a comparison (LT or LR); (b) two studies did not collect data on the study objectives; and (c) four studies were review studies. Ultimately, we included 5 studies with a total of 134 patients comparing the therapeutic effect of LT in the treatment of advanced hepatoblastoma with that of LR in the current meta-analysis [28-32]. All studies were case-series analyses and published in English. The participants were diagnosed with advanced hepatoblastoma according to the PRETEXT system. Among the included studies, all reported both the tumor recurrence rate and the 3-year survival rate [28-32]. Three studies reported the 1-year disease-free survival rate and the 5-year overall or disease-free survival rate [28, 30, 32] and complications [29, 31, 32]. Four studies reported the 1-year survival rate [28-30, 32] and the 3-year disease-free survival rate [28, 30-32]. Table 1 summarizes the demographics, characteristics, and authors of the studies. They include the PRETEXT stage, preoperative chemotherapy, age, sex, tumor status, resection, and prognosis of patients.
According to the search results in the electronic databases, there were no randomized controlled trials on advanced hepatoblastoma patients treated with LT versus LR, and only 5 case series analyses were found. This suggested that these studies had low quality, but it was necessary to use them for a systematic review and meta-analysis because there were no similar reports or consistent therapeutic schedule regimes.
Survival Rate
Most studies (110 patients) reported the 1-year overall survival rate, except for one study [31]. The 1-year overall survival was not significantly different in the treatment of LT versus LR according to the pooled result (OR = 0.89, 95% CI 0.21–3.79, P = 0.88). There was no significant heterogeneity among the studies (P = 0.64, I2 = 0%) (Fig. 2a). All studies (134 patients) reported the 3-year overall survival rate. The 3-year overall survival was not significantly different in the treatment of LT versus LR according to the pooled result (OR = 0.54, 95% CI 0.16–1.81, P = 0.32), and no significant heterogeneity among the studies was found (P = 0.43, I2 = 0%) (Fig. 2b). Three studies (94 patients) reported the 5-year overall survival rate. The 5-year overall survival was not significantly different in the treatment of LT versus LR according to the pooled result (OR = 1.24, 95% CI 0.22–6.82, P = 0.81), and there was no significant heterogeneity among the studies (P = 0.55, I2 = 0%) (Fig. 2c).
Three studies (94 patients) reported the 1-year disease-free survival rate. The 1-year disease-free survival was not significantly different in the treatment of LT versus LR according to the pooled result (OR = 2.17, 95% CI 0.56–8.42, P = 0.26), and no significant heterogeneity among the studies was found (P = 0.32, I2 = 13%) (Fig. 2d). Four studies (118 patients) reported the 3-year disease-free survival rate. The 3-year disease-free survival was not significantly different in the treatment of LT versus LR according to the pooled result (OR = 1.42, 95% CI 0.48–4.17, P = 0.53), and no significant heterogeneity among the studies was found (P = 0.67, I2 = 13%) (Fig. 2e). Three studies (94 patients) reported the 5-year disease-free survival rate. The 5-year disease-free survival was not significantly different in the treatment of LT versus LR according to the pooled result (OR disease-free 5-year = 2.91, 95% CI 0.56–8.52, P = 0.26), and no significant heterogeneity among the studies was found (P = 0.74, I2 = 13%) (Fig. 2f).
Tumor Recurrence, Complications, Publication Bias, and Sensitivity Analysis
All studies (134 patients) reported tumor recurrence. Tumor recurrence was not significantly different in the treatment of LT versus LR according to the pooled result (OR = 0.62, 95% CI 0.24–1.60, P = 0.32), and no significant heterogeneity among the studies was found (P = 0.75, I2 = 0%) (Fig. 3a). Three studies (78 patients) reported complications. The complications of LT had a similar incidence to aggressive LR according to the pooled result (OR = 1.46, 95% CI 0.48–4.49, P = 0.51). There was no significant heterogeneity among the studies (P = 0.58, I2 = 0%) (Fig. 3b).The visual symmetrical plot indicated that the publication bias among the studies was low (Fig. 3c).
To avoid potential bias of the included studies, sensitivity analyses with a fixed- effects model and a random-effects model were performed to assess the therapeutic effects (Table 2). Analysis of LT versus LR showed that LT had similar overall survival rates (1-year P = 0.82, 3-year P = 0.26, 5-year P = 0.90), disease-free survival rate (1-year P = 0.26, 3-year P = 0.56, 5-year P = 0.26), tumor recurrence rate (P = 0.37) and complication rate (P = 0.60) compared with LR.
Discussion
Hepatoblastoma, a rare hepatic disease, is the most frequent intrahepatic malignancy in children and is often diagnosed with a large size and frequent vascular infiltration [33, 34]. The treatment of hepatoblastoma is becoming more successful benefitting from multimodal therapies, including preoperative (neoadjuvant) chemotherapy, surgical resection ensuring removal of the whole tumor and postoperative (adjuvant) chemotherapy [35, 36]. The classic chemotherapy regimen includes a combination of cisplatin and doxorubicin or cisplatin, vincristine and fluorouracil [37, 38]. Poor therapeutic outcomes including radical surgery were common before the 1980s and the introduction of adjunctive therapy, but the resection rate, has been increased from 44 to 70%~85% with the advent of preoperative (neoadjuvant) chemotherapy, turning “unresectable” liver tumors to resectable tumors [28, 39, 40]. Distant metastasis is not a contraindication for the surgical treatment which is consistent with previous trials describing that tumor stage was not an independent risk factor [41, 42]. However, transplant is contraindicated in the presence of any active regional or distant metastatic disease not cleared by chemotherapy or surgery [43].
The most suitable surgical therapy after neoadjuvant therapy is determined by specific tumor characteristics, such as size, critical anatomical localization (e.g., multicenter localization, invasion of vascular/biliary structures) and residual liver function [44, 45]. Different individuals must also be considered. For example, LT should be considered the primary curative option in patients with a risk of relapse, a small-for-size liver remnant and doubtful tumor margins of radical resection [32]. Other generally accepted indications for liver transplantation for hepatoblastoma included the following: (a) unifocal POST-TEXT IV (PRE-TEXT classification following chemotherapy) tumors; (b) POST-TEXT III or IV tumors with persistent widespread multifocality or major vessel involvement; (c) POST-TEXT IV multifocal intrahepatic tumors without metastatic disease after neoadjuvant chemotherapy; (d) PRE-TEXT III or IV tumors, or PRE-TEXT II tumors with poor response to chemotherapy; and (e) POST-TEXT III tumors with major vascular involvement without major vascular reconstruction after conventional resection.
This meta-analysis aimed to assess the effectivity and safety of LT in treating advanced hepatoblastoma in terms of survival, tumor recurrence and complications. Most patients in the included studies were underwent liver transplantation or liver resection after neoadjuvant chemotherapy. According to this meta-analysis, there was no statistically significant effect on the overall or disease-free survival rates after 1 year, 3 years, or 5 years or complications or tumor recurrence between the two surgical schemes. This finding suggested that LT was similar to LR with regard to the survival rate, complications and tumor recurrence. Sensitivity analysis of the current evidence also demonstrated the same trend for LT and LR.
Transplantation had a mean survival rate above 80% with the use of neoadjuvant chemotherapy for unresectable hepatoblastoma, which compared favorably with that after conventional LR (Table 1). Some recent studies also suggested that the tumor recurrence rate after LT is lower than that after LR [29, 30, 32]. The advantages of LT in increasing disease-free survival and reducing recurrence may be as follows [46, 47]: (a) LT, as a more aggressive surgical approach, can avoid microscopic residual disease; and (b) LT reduces the risk of tumor metastasis by reducing tumor squeeze. However, these advantages did not seem to have significant indication according to the current meta-analysis. The reason for this may be as follows: (a) All tumors had already achieved complete resection in the included studies; (b) Most LT cases were more advanced than LR cases in the included studies; and (c) The included studies were limited. Chemotherapy should also be taken into account. Moreover, in consideration of surgical complications, there are some conflicting views [23, 48-50]. According to published studies, the common complications of LT are biliary complications, hemorrhage and portal vein thrombosis, but biliary complications and hemorrhage are also common after LR [50, 51]. The surgical complications in this meta-analysis were consistent with the published data. However, a detailed complication analysis was not performed in this meta-analysis due to a lack of sufficient data. Considering that limited case series analyses were available and included in this paper, well-designed and powered double-blinded RCTs comparing LT with LR are needed to confirm and provide solid clinical evidence on the benefits, recurrence and complication profiles of LT over LR.
We acknowledge that only five case series analyses were available and included in this meta-analysis, without high-quality RCTs. The main reason for this is that it remains a challenge to conduct clinical research trials with randomization and double blinding because of the limited cases. Both are effective means of preventing bias and improving the objectivity of clinical evidence for both the efficacy and safety of any approved medical product or procedure, device or therapeutic regimen [52]. Furthermore, the other limitations were as follows: (a) This study was a case series analysis with inherent selection bias; (b) The detailed chemotherapy protocol and its complications should be described in the study; and (c) Some statistical limitations associated with the case series analyses must be considered, including the differences among institutions in perioperative management, unstandardized chemotherapy regimens, and the small number of patients. Thus, further large randomized clinical trials or large-scale comparative studies are needed to investigate the outcomes of survival, tumor recurrence and complications. However, the meta-analysis seems to indicate that LT shows excellent therapeutic outcomes in pediatric advanced hepatoblastoma. Considering that the data on hepatoblastoma are not widely published, we believe that our study adds to previously published research.
In conclusion, this meta-analysis indicates that LT, as curative resection, shows excellent outcomes which are not inferior to LR in pediatric advanced hepatoblastoma. And LT should be considered the primary curative option in cases of advanced hepatoblastoma when LR is not possible, which needs to be further confirmed by high-quality RCTs. To select the most optimal surgical regimen for each patient with advanced hepatoblastoma, we want to highlight the necessity of a detailed assessment by an experienced interdisciplinary team in a center for transplant surgery, hepato-pancreatico-biliary surgery and pediatric surgery.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Hubbard AK, Spector LG, Fortuna G, Marcotte EL, Poynter JN (2019) Trends in international incidence of pediatric cancers in children under 5 years of age: 1988–2012. JNCI Cancer Spectr 3:pkz007
Allan BJ, Parikh PP, Diaz S, Perez EA, Neville HL, Sola JE (2013) Predictors of survival and incidence of hepatoblastoma in the paediatric population. HPB (Oxford) 15:741–746
Pham TA, Gallo AM, Concepcion W, Esquivel CO, Bonham CA (2015) Effect of liver transplant on long-term disease-free survival in children with hepatoblastoma and hepatocellular cancer. JAMA Surg 150:1150–1158
Reynolds M, Douglass EC, Finegold M, Cantor A, Glicksman A (1992) Chemotherapy can convert unresectable hepatoblastoma. J Pediatr Surg 27:1080–1084
Khaderi S, Guiteau J, Cotton RT, O’mahony C, Rana A, Goss JA (2014) Role of liver transplantation in the management of hepatoblastoma in the pediatric population. World J Transplant 4:294–298
Kremer N, Walther AE, Tiao GM (2014) Management of hepatoblastoma: an update. Curr Opin Pediatr 26:362–369
Towbin AJ, Meyers RL, Woodley H et al (2018) 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol 48:536–554
Maibach R, Roebuck D, Brugieres L et al (2012) Prognostic stratification for children with hepatoblastoma: the SIOPEL experience. Eur J Cancer 48:1543–1549
Feng TC, Zai HY, Jiang W et al (2019) Survival and analysis of prognostic factors for hepatoblastoma: based on SEER database. Ann Transl Med 7:555
Otte JB, Pritchard J, Aronson DC et al (2004) Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer 42:74–83
Browne M, Sher D, Grant D et al (2008) Survival after liver transplantation for hepatoblastoma: a 2-center experience. J Pediatr Surg 43:1973–1981
Ismail H, Broniszczak D, Kaliciński P et al (2012) Changing treatment and outcome of children with hepatoblastoma: analysis of a single center experience over the last 20 years. J Pediatr Surg 47:1331–1339
Katzenstein HM, London WB, Douglass EC et al (2002) Treatment of unresectable and metastatic hepatoblastoma: a pediatric oncology group phase II study. J Clin Oncol 20:3438–3444
Perilongo G, Maibach R, Shafford E et al (2009) Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med 361:1662–1670
Hiyama E, Hishiki T, Watanabe K et al (2020) Outcome and late complications of hepatoblastomas treated using the Japanese study group for pediatric liver tumor 2 protocol. J Clin Oncol 38:2488–2498
Lim IIP, Bondoc AJ, Geller JI, Tiao GM (2018) Hepatoblastoma-the evolution of biology, surgery, and transplantation. Children (Basel) 6:1
Dicken BJ, Bigam DL, Lees GM (2004) Association between surgical margins and long-term outcome in advanced hepatoblastoma. J Pediatr Surg 39:721–725
Fuchs J, Rydzynski J, Von Schweinitz D et al (2002) Pretreatment prognostic factors and treatment results in children with hepatoblastoma: a report from the German Cooperative Pediatric Liver Tumor Study HB 94. Cancer 95:172–182
Casas-Melley AT, Malatack J, Consolini D et al (2007) Successful liver transplant for unresectable hepatoblastoma. J Pediatr Surg 42:184–187
Sakamoto S, Kasahara M, Mizuta K et al (2014) Nationwide survey of the outcomes of living donor liver transplantation for hepatoblastoma in Japan. Liver Transpl 20:333–346
Ramos-Gonzalez G, Laquaglia M, O’neill AF et al (2018) Long-term outcomes of liver transplantation for hepatoblastoma: a single-center 14-year experience. Pediatr Transplant e13250
Fuchs J, Cavdar S, Blumenstock G et al (2017) POST-TEXT III and IV Hepatoblastoma: extended hepatic resection avoids liver transplantation in selected cases. Ann Surg 266:318–323
Lautz TB, Ben-Ami T, Tantemsapya N, Gosiengfiao Y, Superina RA (2011) Successful nontransplant resection of POST-TEXT III and IV hepatoblastoma. Cancer 117:1976–1983
Wu T, Liu G (2007) The concepts, design, practice and reports of allocation concealment and blinding. Chin J Evid Based Med 7:e225
Li JZ, Dong ZY, Zhang XJ, Huang CY, Xu J (2017) Transcatheter arterial chemoembolization in combination with stereotactic body radiation therapy in primary liver carcinoma a systematic review and meta-analysis. Int J Clin Exp Med 10:1816–1827
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Bmj 327:557–560
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Pimpalwar AP, Sharif K, Ramani P et al (2002) Strategy for hepatoblastoma management: transplant versus nontransplant surgery. J Pediatr Surg 37:240–245
Caicedo LA, Sabogal A, Serrano O et al (2017) Hepatoblastoma: transplant versus resection experience in a Latin American transplant center. Transplant Direct 3:e165
Uchida H, Sakamoto S, Sasaki K et al (2018) Surgical treatment strategy for advanced hepatoblastoma: resection versus transplantation 65:e27383
De Freitas PG, Tannuri ACA, Dantas Marques AC, Torres RR, Mendes Gibelli NE, Tannuri U (2019) Extensive hepatectomy as an alternative to liver transplant in advanced hepatoblastoma: a new protocol used in a pediatric liver transplantation center. Transplant Proc 51:1605–1610
Moosburner S, Schmelzle M, Schöning W, Kästner A, Seika P, Globke B (2021) Liver transplantation is highly effective in children with irresectable hepatoblastoma. Medicina (Kaunas) 57:819
Shi Y, Commander SJ, Masand PM, Heczey A, Goss JA, Vasudevan SA (2017) Vascular invasion is a prognostic indicator in hepatoblastoma. J Pediatr Surg 52:956–961
Sharma D, Subbarao G, Saxena R (2017) Hepatoblastoma. Semin Diagn Pathol 34:192–200
Hafberg E, Borinstein SC, Alexopoulos SP (2019) Contemporary management of hepatoblastoma. Curr Opin Organ Transplant 24:113–117
Ezekian B, Mulvihill MS, Schroder PM et al (2018) Improved contemporary outcomes of liver transplantation for pediatric hepatoblastoma and hepatocellular carcinoma. Pediatr Transplant 22:e13305
Zsiros J, Brugieres L, Brock P et al (2013) Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol 14:834–842
Katzenstein HM, Malogolowkin MH, Krailo MD et al (2022) Doxorubicin in combination with cisplatin, 5-flourouracil, and vincristine is feasible and effective in unresectable hepatoblastoma: a Children's Oncology Group study. Cancer 128:1057–1065
Evans AE, Land VJ, Newton WA, Randolph JG, Sather HN, Tefft M (1982) Combination chemotherapy (vincristine, adriamycin, cyclophosphamide, and 5-fluorouracil) in the treatment of children with malignant hepatoma. Cancer 50:821–826
Nguyen R, Mccarville MB, Sykes A et al (2018) Rapid decrease of serum alpha-fetoprotein and tumor volume predicts outcome in children with hepatoblastoma treated with neoadjuvant chemotherapy. Int J Clin Oncol 23:900–907
Angelico R, Grimaldi C, Gazia C et al (2019) How do synchronous lung metastases influence the surgical management of children with hepatoblastoma? An update and systematic review of the literature. Cancers (Basel) 11:1693
Lake CM, Tiao GM, Bondoc AJ (2019) Surgical management of locally-advanced and metastatic hepatoblastoma. Semin Pediatr Surg 28:150856
Trobaugh-Lotrario AD, Meyers RL, Tiao GM, Feusner JH (2016) Pediatric liver transplantation for hepatoblastoma. Transl Gastroenterol Hepatol 1:44
Meyers RL, Tiao GM, Dunn SP, Mcgahren ED 3rd, Langham MR Jr (2012) Surgical management of children with locally advanced hepatoblastoma. Cancer 118:4090–4095
Otte JB, Meyers RL, De Ville De Goyet J (2013) Transplantation for liver tumors in children: time to (re)set the guidelines? Pediatr Transplant 17:710–712
Younes A, Elgendy A (2021) Surgical resection of hepatoblastoma: factors affecting local recurrence 31:432–438
Li F, Zhang W, Hu H, Zhu X, Zhang Y, Huang D (2021) Factors influencing recurrence after complete remission in children with hepatoblastoma: a 14-year retrospective study in China. PLoS One 16:e0259503
Shin M, Song S, Kim JM et al (2012) Donor morbidity including biliary complications in living-donor liver transplantation: single-center analysis of 827 cases. Transplantation 93:942–948
Iwasaki J, Iida T, Mizumoto M et al (2014) Donor morbidity in right and left hemiliver living donor liver transplantation: the impact of graft selection and surgical innovation on donor safety. Transpl Int 27:1205–1213
Abecassis MM, Fisher RA, Olthoff KM et al (2012) Complications of living donor hepatic lobectomy–a comprehensive report. Am J Transplant 12:1208–1217
Trobaugh-Lotrario AD, Meyers RL, O’neill AF, Feusner JH (2017) Unresectable hepatoblastoma: current perspectives. Hepat Med 9:1–6
Imai Y (2010) Combination of transarterial chemoembolization and percutaneous local ablation therapy for hepatocellular carcinoma. Hepatol Res 40:105–107
Acknowledgements
We thank Zhiyong Dong for editing our manuscript, which substantially improved the quality of the article.
Funding
This work was supported by the Healthcare Appropriate Technology Development and Promotion Project of Guangxi (No.S2018086) grant.
Author information
Authors and Affiliations
Contributions
Yanhua Lai performed literature search and collecting data and drafted the manuscript.
Dan Wu participated in literature search and collecting data. Ruihua Deng participated in literature search. Jiazhi Li performed the statistical analysis, study design, literature search, original draft preparation and revision and participated in its design. Jianrong Yang revised the manuscript, performed previous study design and supplemental data. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lai, Y., Wu, D., Deng, R. et al. Liver Transplantation in Children with Advanced Hepatoblastoma: a Systematic Review and Meta-Analysis. Indian J Surg 86, 64–72 (2024). https://doi.org/10.1007/s12262-023-03839-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-023-03839-4