Abstract
Graves’ disease (GD) is a systemic autoimmune disorder mainly affecting the thyroid gland. During GD management, the principal target is to control the hyperthyroid state. There have been three rather similarly effective modalities: medical therapy with antithyroid drugs (ATD), radioactive iodine (RAI), or surgical excision of the thyroid tissue (thyroidectomy). Defining the relative risks and benefits of each of the two potential definitive treatment options (RAI or thyroidectomy) is crucial for creating evidence-based therapy algorithms. This systematic review and meta-analysis aimed to compare the outcomes of these two treatment options. This is a systematic review and meta-analysis that analyzed the studies comparing RAI and thyroidectomy to treat GD. Studies were obtained by searching on Scopus, the Cochrane Central Register of Controlled Trials, and PubMed central database. The surgically treated group showed significantly lower failure rates, non-significantly lower cardiovascular morbidities, non-significantly higher complication rates, and significantly lower mortality rates. The RAI-related complications were mostly the development or worsening of Graves’ ophthalmopathy. This review and meta-analysis comparing surgery and radioactive iodine for the treatment of Grave’s disease from 16 well-conducted trials has shown that although surgery viz., total thyroidectomy was less frequently utilized for the treatment of Grave’s disease, it controlled the symptoms with greater success and without any worsening of Grave’s ophthalmopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graves’ disease (GD) is a systemic autoimmune disorder mainly affecting the thyroid gland. It is the most prevalent cause of hyperthyroidism and has been reported to affect about 1–1.5% of the world population [1]. Graves’ disease affects people of all ages, but notably middle-aged women [2].
Graves’ disease develops as a result of thyroid-stimulating immunoglobulin (TSI) impact on the thyroid-stimulating hormone receptors (TSHR), leading to excessive secretion of thyroxin and loss of pituitary feedback control on the thyroid gland [3]. Thyroid-stimulating immunoglobulins also contribute to Graves’ orbitopathy (GO) development through their effect on retro-orbital tissue thyrotropin receptors [2]. Although GD is primarily predisposed by genetic causes, environmental causes, including smoking, vitamin D deficiency, and iodine excess, contribute to the occurrence of the disease [4].
During GD management, the principal target is to control the hyperthyroid state by normalization of the thyroid hormone concentration. The presence of GO and/or a goiter (enlarged nodular thyroid gland) will influence the choice of therapy [2]. There have been three rather similarly effective modalities: medical therapy with antithyroid drugs (ATD) that inhibit the thyroid hormone production, radioactive iodine (RAI) for induction of thyroid tissue shrinkage, or surgical excision of the thyroid tissue (thyroidectomy) [5].
The choice of either treatment approach is an issue of wide debate. While thyroidectomy or ATD is the favorable choice in Europe, RAI is the preferred modality in the USA [6,7,8,9,10]. Indeed, the current guidelines have assumed surgical management as an equivalent therapy choice to RAI, with a comparable long-term outcome regarding the quality of life [8, 11, 12]. Moreover, some studies have recently shown that thyroidectomy is the definitive treatment of choice [13,14,15].
Radioactive iodine is indicated in patients with persistent thyrotoxicosis despite a course of ATD for 1–1.5 years, patients with recurrent or relapsed hyperthyroidism, those who are not candidates for ATDs, and those who prefer this modality. It is contraindicated in pregnant and lactating women, in cases of GO, nodular goiter, or suspected thyroid malignancy [8, 16,17,18].
Thyroidectomy is indicated in patients with persistent, recurrent, or relapsed thyrotoxicosis after completing the ATDs course, those with goiter, suspected thyroid malignancy, active GO, and pregnant females during the second trimester [8, 17,18,19,20]. Defining the relative risks and benefits of each of the two potential definitive treatment options (RAI or thyroidectomy) is crucial for creating evidence-based therapy algorithms. This systematic review and meta-analysis aimed to compare the outcomes of these two treatment options.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed when conducting this study [20].
Selection Strategy and Criteria
The following electronic resources were searched for the required articles: the Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, and the PubMed central database.
Two independent researchers (the first and second authors) conducted the search using the following keywords: Graves’ disease, primary hyperthyroidism, radioactive iodine, operative treatment, and surgery. Only original works published in English were included in the search. The acquired articles were screened, and then they underwent an eligibility check.
Inclusion Criteria
Original data comparing the outcomes of surgery and RAI for the treatment of adult patients with Graves’ disease were eligible for the study.
Exclusion Criteria
Reviews, commentaries, and letters to the editor were excluded. Studies that did not compare operative treatment and RAI in terms of clinical outcome, efficacy, and safety; studies that did not discriminate cases of Graves’ disease from other causes of hyperthyroidism; and those involving pediatric patients were also excluded.
Data Extraction, Collection, and Analysis
The included articles were assessed, and the data related to the search was extracted and analyzed. The included studies were evaluated for the bias encountered using the “Cochrane Collaboration tool for assessing the risk of bias.”
Summary Measures
The primary outcomes were the difference between the two operations in the perioperative data, the postoperative complications, and the rate of recurrence. The secondary outcomes were the difference in the quality of life (QoL) and the satisfaction rate.
Statistical Analysis
The collected data were tabulated and analyzed. Using the Review Manager Software (RevMan version 5.4, the Cochrane Collaboration, London, United Kingdom), the meta-analysis tests and bias evaluation were carried out. The categorical data were expressed as risk ratios and 95% confidence intervals (CIs), and the numerical data were compared with the differences in means of the effects between the two groups. The I2 statistic’s indication of data heterogeneity led to the use of random or fixed-effect models for the analysis.
Results
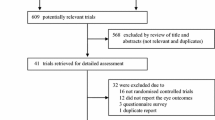
Initially, researching the electronic resources yielded 2151 records. The remaining records after adjusting for the duplications were 382. After checking the articles’ titles and abstracts, an additional 311 articles were excluded. Reading the full texts of the remaining 71 articles resulted in the exclusion of 59 articles. Research in the references of the remaining included articles yielded an additional 4 studies. Finally, sixteen articles were eligible for this analysis [12, 21,22,23,24,25,26,27,28,29,30,31,32,33,34,35], of which only one was a randomized controlled trial (RCT) [23], two were prospective studies [22, 25], and the remaining thirteen were retrospective [12, 21, 24, 26,27,28,29,30,31,32,33,34,35]. The review flow chart is shown in Fig. 1.
The publications included in this analysis covered around four decades of research and were published between 1980 and 2022. Patients with Graves’ disease who were scheduled for surgery or RAI made up the study population. The included studies’ sample sizes ranged from 13 [25] to 1844 [31], with a total population of 8395. Of those, 2010 (23.9%) patients were treated surgically, and 6385 (76.1%) were treated with RAI. The patients were followed for anything between 6.8 and 94 months (Tables 1 and 2).
In terms of the study outcomes, these focused mostly on the difference between groups in the relapse/persistency of hyperthyroidism and post-treatment complications. The baseline demographic parameters of the patients were generally comparable in the two groups.
The meta-analysis of 13 studies [12, 21,22,23,24,25,26,27,28, 31,32,33,34] that evaluated the post-treatment failure (persistence or relapse) demonstrated higher failure recurrence rates in patients treated with RAI compared to those treated surgically (12.7% vs. 3.1%) with a statistically significant difference (p = 0.03) (Fig. 2).
Postoperative complication rates were described in 8 studies [23, 25,26,27,28, 30, 31, 34]. Higher rates were noted in the surgically treated group, with non-statistically significant differences (p = 0.07) (Fig. 3). Most of the surgery-related complications were transient and easily controlled, while the majority of complications in the RAI group were developing or worsening orbitopathy (80.5%). Cardiovascular complications were non-significantly higher in the RAI group [12, 30, 32, 35] without statistical significance (p = 0.1) (Fig. 4).
Only 2 studies mentioned the rates of mortality [32, 34]. Their analysis revealed statistically higher RAI-associated mortality (10.8 vs. 3.05, p = 0.03) (Fig. 5). The long-term QoL outcome was investigated in two studies [12, 29]. Both concluded that patients undergoing treatment for Graves’ hyperthyroidism had worse QoL in comparison with the general population. One of them stated that the type of treatment for GD, whether medical, surgical or by RAI, has little effect on the long-term QoL [12], while the other established that patients treated with RAI had worsened long-term thyroid-specific and general QoL [29].
Risks of Bias
The figure displays the review authors’ critical evaluation of the studies’ potential for bias and presents their conclusions as percentages across all included studies for each risk of bias item. The primary bias in the included studies was a result of selection and performance bias, which was built into the study design (Fig. 6).
Discussion
Worldwide, GD is the most frequent cause of adult-onset persistent hyperthyroidism. Treatment of Graves’ disease is influenced by institutional-based practices, surgical considerations, and the dominance of endocrinologists or nuclear physicians. Moreover, referral for surgical intervention is likely affected by the financial, cultural, and preferences of the physician or the patient.
Thus, a proper decision should be based on dedicated knowledge of the benefits and risks of each choice. Only one systematic review could be found comparing the treatment options for Graves’ disease [36]. This was published in 2013, since when some original research studies have been published addressing this topic. Hence, we conducted this analysis in an attempt to provide a summary of the latest available evidence investigating the pros and cons of each treatment option for GD. More specifically, management by thyroidectomy and RAI, being the definitive treatment for GD, was compared in this analysis.
This study demonstrated that surgical treatment overall had a better outcome, with a significantly lower failure rate. These findings are congruent with the previously reported data [36, 37]. Although the surgical option was associated with more frequent complications, most of these complications were transient. Moreover, surgery was associated with statistically less mortality. Despite being drawn from the analysis of two studies only, the significantly higher RAI-related mortality must not be overlooked.
The majority of the RAI-related complications in this analysis were the de novo development or worsening of Grave’s ophthalmopathy. This is in line with the meta-analysis conducted by Li et al. [38] which concluded that RAI treatment resulted in a higher risk of the occurrence or worsening of GO. This may be explained by the abrupt elevation of TSH receptor antibodies during the first 6 months after treatment with RAI [39].
RAI was associated with a significantly higher long-term incidence of cardiovascular morbidity. Radiation has been shown to accelerate atherosclerosis and induce reactive oxygen species formation, which is crucial in the development and worsening of cardiovascular disease [40]. Adopting RAI as favored management has emerged following its initial introduction in the 1900s due to the surgery-associated high complication rates and costs at that time. Nevertheless, currently, the pendulum is likely turning back to the re-consideration of operative treatment for GD. It has been assumed that RAI may lead to higher malignancy rates [41].
The thyroidectomy option is advantageous by treating GD immediately and definitively with avoidance of the risks related to the long-term administration of ATD or RAI. However, thyroidectomy is the least preferred choice for hyperthyroidism treatment [42,43,44,45]. This is attributed to concerns regarding the potential postoperative complications, such as recurrent laryngeal nerve (RLN) injury, hypoparathyroidism, as well as permanent hypothyroidism, and neck scarring.
Advances in operative procedures, including the remote-access thyroidectomy and intraoperative monitoring of the RLN, together with the present awareness of the RAI-associated risks, raise the need to shed light on thyroidectomy as the main definite treatment for GD, by considering RAI only for cases where patients with GD are contraindicated to surgery.
This study is strengthened by summing up evidence driven by studies comparing the two treatment options in a large cohort over about 4 decades, extending until the year 2022. The work is limited by that most of the included studies were retrospective analyses. The study is also limited by not addressing the effect of the number of RAI doses or the extent of thyroidectomy on the outcome. However, we think that this is beyond the scope of this study.
Conclusion
This review and meta-analysis comparing surgery and radioactive iodine for the treatment of Grave’s disease from 16 well-conducted trials has shown that although surgery viz. total thyroidectomy was less frequently utilized for the treatment of Grave’s disease, it controlled the symptoms with greater success and without any worsening of Grave’s ophthalmopathy.
References
Smith TJ, Hegedüs L (2016) Graves’ Disease. N Engl J Med 375:1552–1565. https://doi.org/10.1056/NEJMra1510030
Girgis CM, Champion BL, Wall JR (2011) Current concepts in Graves’ disease. Ther Adv Endocrinol Metab 2:135–144. https://doi.org/10.1177/2042018811408488
Antonelli A, Ferrari SM, Ragusa F et al (2020) Graves’ disease: epidemiology, genetic and environmental risk factors, and viruses. Best Pract Res Clin Endocrinol Metab 34:101387. https://doi.org/10.1016/j.beem.2020.101387
Kahaly GJ (2020) Management of Graves thyroidal and extrathyroidal disease: an update. J Clin Endocrinol Metab 105:dgaa646. https://doi.org/10.1210/clinem/dgaa646
Davies TF, Andersen S, Latif R et al (2020) Graves’ disease. Nat Rev Dis Primers 6:1–23. https://doi.org/10.1038/s41572-020-0184-y
Lal G, Ituarte P, Kebebew E et al (2005) Should total thyroidectomy become the preferred procedure for surgical management of Graves’ disease? Thyroid 15:569–574. https://doi.org/10.1089/thy.2005.15.569
Schneider DF, Sonderman PE, Jones MF et al (2014) Failure of radioactive iodine in the treatment of hyperthyroidism. Ann Surg Oncol 21:4174–4180. https://doi.org/10.1245/s10434-014-3858-4
Bahn RS, Burch HB, Cooper DS et al (2011) Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract 17:456–520. https://doi.org/10.4158/ep.17.3.456
Wartofsky L, Glinoer D, Solomon B et al (1991) Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid 1:129–135. https://doi.org/10.1089/thy.1991.1.129
Werga-Kjellman P, Zedenius J, Tallstedt L et al (2001) Surgical treatment of hyperthyroidism: a ten-year experience. Thyroid 11:187–192. https://doi.org/10.1089/105072501300042947
Wong KK, Shulkin BL, Gross MD, Avram AM (2018) Efficacy of radioactive iodine treatment of graves’ hyperthyroidism using a single calculated 131I dose. Clin Diabetes Endocrinol 4:20. https://doi.org/10.1186/s40842-018-0071-6
Abraham-Nordling M, Törring O, Hamberger B et al (2005) Graves’ disease: a long-term quality-of-life follow up of patients randomized to treatment with antithyroid drugs, radioiodine, or surgery. Thyroid 15:1279–1286. https://doi.org/10.1089/thy.2005.15.1279
Burch HB, Cooper DS (2015) Management of Graves disease: a review. JAMA 314:2544–2554. https://doi.org/10.1001/jama.2015.16535
Elfenbein DM, Schneider DF, Havlena J et al (2015) Clinical and socioeconomic factors influence treatment decisions in Graves’ disease. Ann Surg Oncol 22:1196–1199. https://doi.org/10.1245/s10434-014-4095-6
Jin J, Sandoval V, Lawless ME et al (2012) Disparity in the management of Graves’ disease observed at an urban county hospital: a decade-long experience. Am J Surg 204:199–202. https://doi.org/10.1016/j.amjsurg.2011.10.010
Kahaly GJ, Bartalena L, Hegedüs L et al (2018) 2018 European Thyroid Association guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J 7:167–186. https://doi.org/10.1159/000490384
Bartalena L (2013) Diagnosis and management of Graves disease: a global overview. Nat Rev Endocrinol 9:724–734. https://doi.org/10.1038/nrendo.2013.193
Ross DS, Burch HB, Cooper DS et al (2016) 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26:1343–1421. https://doi.org/10.1089/thy.2016.0229
De Leo S, Lee SY, Braverman LE (2016) Hyperthyroidism. Lancet 388:906–918. https://doi.org/10.1016/S0140-6736(16)00278-6
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Sugrue D, McEvoy M, Feely J, Drury MI (1980) Hyperthyroidism in the land of Graves: results of treatment by surgery, radio-iodine and carbimazole in 837 cases. Q J Med 49:51–61
Berglund J, Christensen SB, Dymling JF, Hallengren B (1991) The incidence of recurrence and hypothyroidism following treatment with antithyroid drugs, surgery, or radioiodine in all patients with thyrotoxicosis in Malmö during the period 1970–1974. J Intern Med 229:435–442. https://doi.org/10.1111/j.1365-2796.1991.tb00371.x
Törring O, Tallstedt L, Wallin G et al (1996) Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine–a prospective, randomized study. Thyroid Study Group. J Clin Endocrinol Metab 81:2986–2993. https://doi.org/10.1210/jcem.81.8.8768863
Leary AC, Grealy G, Higgins TM et al (1999) Long-term outcomes of treatment of hyperthyroidism in Ireland. Ir J Med Sci 168:47–52. https://doi.org/10.1007/BF02939582
Tütüncü NB, Tütüncü T, Ozgen A, Erbas T (2006) Long-term outcome of Graves’ disease patients treated in a region with iodine deficiency: relapse rate increases in years with thionamides. J Natl Med Assoc 98:926–930
Kautbally S, Alexopoulou O, Daumerie C et al (2012) Greater efficacy of total thyroidectomy versus radioiodine therapy on hyperthyroidism and thyroid-stimulating immunoglobulin levels in patients with Graves’ disease previously treated with antithyroid drugs. Eur Thyroid J 1:122–128. https://doi.org/10.1159/000339473
Sundaresh V, Brito JP, Thapa P et al (2017) Comparative effectiveness of treatment choices for Graves’ hyperthyroidism: a historical cohort study. Thyroid 27:497–505. https://doi.org/10.1089/thy.2016.0343
Wu VT, Lorenzen AW, Beck AC et al (2017) Comparative analysis of radioactive iodine versus thyroidectomy for definitive treatment of Graves disease. Surgery 161:147–155. https://doi.org/10.1016/j.surg.2016.06.066
Törring O, Watt T, Sjölin G et al (2019) Impaired quality of life after radioiodine therapy compared to antithyroid drugs or surgical treatment for Graves’ hyperthyroidism: a long-term follow-up with the thyroid-related patient-reported outcome questionnaire and 36-item short form health status survey. Thyroid 29:322–331. https://doi.org/10.1089/thy.2018.0315
Gibson A, Dave A, Johnson C et al (2020) Cardiovascular outcomes of thyroidectomy or radioactive iodine ablation for Graves’ disease. J Surg Res 256:486–491. https://doi.org/10.1016/j.jss.2020.07.020
Brito JP, Payne S, Singh Ospina N et al (2020) Patterns of use, efficacy, and safety of treatment options for patients with Graves’ disease: a nationwide population-based study. Thyroid 30:357–364. https://doi.org/10.1089/thy.2019.0132
Liu X, Wong CKH, Chan WWL et al (2021) Outcomes of Graves’ disease patients following antithyroid drugs, radioactive iodine, or thyroidectomy as the first-line treatment. Ann Surg 273:1197–1206. https://doi.org/10.1097/SLA.0000000000004828
Kim MJ, Kim YA, Cho SW et al (2021) Secular trends in ablation therapy for Graves’ disease: an analysis of a 15-year experience at a tertiary hospital in South Korea. J Clin Med 10:1629. https://doi.org/10.3390/jcm10081629
Thewjitcharoen Y, Karndumri K, Chatchomchuan W et al (2021) Practice patterns and outcomes in the management of Thai patients with Graves’ disease. Thyroid Res 14:5. https://doi.org/10.1186/s13044-021-00097-y
Liu X, Wong CKH, Chan WWL et al (2022) Long-term outcome of patients treated with antithyroid drugs, radioactive iodine or surgery for persistent or relapsed Graves’ disease. Br J Surg 109:381–389. https://doi.org/10.1093/bjs/znab474
Sundaresh V, Brito JP, Wang Z et al (2013) Comparative effectiveness of therapies for Graves’ hyperthyroidism: a systematic review and network meta-analysis. J Clin Endocrinol Metab 98:3671–3677. https://doi.org/10.1210/jc.2013-1954
Genovese BM, Noureldine SI, Gleeson EM et al (2013) What is the best definitive treatment for Graves’ disease? A systematic review of the existing literature. Ann Surg Oncol 20:660–667. https://doi.org/10.1245/s10434-012-2606-x
Li HX, Xiang N, Hu WK, Jiao XL (2016) Relation between therapy options for Graves’ disease and the course of Graves’ ophthalmopathy: a systematic review and meta-analysis. J Endocrinol Invest 39:1225–1233. https://doi.org/10.1007/s40618-016-0484-y
Wiersinga WM (2019) Graves’ disease: can it be cured? Endocrinol Metab 34:29–38. https://doi.org/10.3803/EnM.2019.34.1.29
Sylvester CB, Abe J, Patel ZS, Grande-Allen KJ (2018) Radiation-induced cardiovascular disease: mechanisms and importance of linear energy transfer. Front Cardiovasc Med 5:5. https://doi.org/10.3389/fcvm.2018.00005
Shim SR, Kitahara CM, Cha ES et al (2021) Cancer risk after radioactive iodine treatment for hyperthyroidism: a systematic review and meta-analysis. JAMA Network Open 4:e2125072. https://doi.org/10.1001/jamanetworkopen.2021.25072
Koren S, Shteinshnaider M, Or K et al (2019) A 2017 survey of the clinical practice patterns in the management of relapsing Graves disease. Endocr Pract 25:55–61. https://doi.org/10.4158/EP-2018-0386
Bartalena L, Burch HB, Burman KD, Kahaly GJ (2016) A 2013 European survey of clinical practice patterns in the management of Graves’ disease. Clin Endocrinol 84:115–120. https://doi.org/10.1111/cen.12688
Burch HB, Burman KD, Cooper DS (2012) A 2011 survey of clinical practice patterns in the management of Graves’ disease. J Clin Endocrinol Metab 97:4549–4558. https://doi.org/10.1210/jc.2012-2802
Cohen O, Ronen O, Khafif A et al (2022) Revisiting the role of surgery in the treatment of Graves’ disease. Clin Endocrinol 96:747–757. https://doi.org/10.1111/cen.14653
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salman, M.A., Assal, M.M., Salman, A. et al. Outcomes of Radioactive Iodine Versus Surgery for the Treatment of Graves’ Disease: a Systematic Review and Meta-analysis. Indian J Surg (2023). https://doi.org/10.1007/s12262-023-03692-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12262-023-03692-5