Abstract
Closing the midline in patients with incisional hernias is the cornerstone for a functional reconstruction with low morbidity, low recurrence rates, and good cosmetic results, which is the ideal outcome for every hernia surgeon. However, in patients with large hernias (usually over 10 cm width) or in loss of domain cases, this goal is difficult to achieve. Anterior component separation with or without mesh reinforcement has been the procedure of choice for these patients despite its high rate of wound complications. The goal of our study is to evaluate the opportunity and necessity of the anterior component separation in patients with complex incisional or ventral hernias (defects larger than 10 cm, infected meshes). Data of patients with large incisional/ventral hernia operated using anterior component separation technique in the past 10 years were re-visited and analyzed from hospital records between January 2012 and December 2020. Demographic data (age, gender, body mass index, ASA score) and the main steps of the technique were recorded. Data were reported as mean and standard deviation. We used the anterior component separation in 66 cases, mainly for septic conditions (open abdomen, chronic and extended infections of the abdominal wall, chronic-infected meshes). For large parietal defects with aseptic local condition, we used mesh-reinforced anterior component separation (five patients). Mean age was 68.7 years. Among them, 29 patients developed wound complications (hematoma, seroma, infection). Mean hospital stay was 12.6 days. Recurrence was 18% in patients without mesh and zero in patients with mesh reinforcement after a minimum one-year follow-up. Anterior component separation is still a valid procedure in patients with large abdominal defects especially when a septic wound is to be closed. For large parietal defects, if a wide subcutaneous dissection is required, mesh-reinforced anterior component separation remains a valid alternative in abdominal wall reconstruction in certain cases (mainly aseptic conditions).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of hernias following laparotomy ranges between 9 and 50% in high-risk population patients. There also is an increased number of complex incisional hernias (IH) most of them featuring large defects (usually over 10 cm width) or loss of domain, which can lead to a profound alteration of the anatomy and function of the abdominal wall [1]. Once the linea alba has been broken, the resulting scar tissue is weaker, leading to hernia formation in specific circumstances. In recent years, the concept of abdominal wall reconstruction has been a hot topic in the field of hernia surgery. The goal of this reconstruction is a mesh-reinforced primary fascial closure, with reconstruction of the midline (linea alba) under minimal tension, resulting in minimal complications. Thus, the procedure provides the patient with both a sound and cosmetic result.
Sometimes, due to excessive tension or severe postoperative complications generated by the forced repair (intra-abdominal hypertension, abdominal compartment syndrome, or respiratory failure), large defects or loss of domain hernias cannot be closed without disconnecting some of the components of the lateral abdominal wall. These techniques take advantage of the layered anatomy of the abdominal wall, and, based on which layers are released, they can be categorized as anterior or posterior component separation.
We set out to analyze whether mesh-reinforced anterior component separation (MACS) should remain in the therapeutic arsenal and to exemplify instances in our cases when this procedure may be recommended.
Patients and Methods
Patients
We reviewed all the files of the patients with complex incisional hernias (defects larger than 10 cm width, infected abdominal wall meshes, open septic abdomen, extended infections of the abdominal wall) and ACS with and without mesh reinforcement admitted in both departments of surgery during January 2012 and December 2020 after institutional board approval. Demographic variables including patient’s age and gender were collected. Medical co-morbidities such as body mass index (BMI), chronic obstructive pulmonary disease (COPD), diabetes, smoking and alcohol habits, and ASA score were also reported. All admitted patients were investigated with standard abdominal ultrasound and native or contrast-enhanced abdomino-pelvic CT scan for size and location of the defect, muscular status, presence or absence of mesh, and infected mesh. The authors, both senior surgeons experienced in open and laparoscopic abdominal wall reconstruction, performed all the procedures.
Surgical Technique
Patients were admitted to hospital the night before surgery and received an osmotic bowel preparation and the prophylaxis of deep vein thrombosis with low molecular weight heparins according to the anesthesiologist preference. The exact size and position of the defect relative to the anatomic midline and level of the umbilicus were noted. The surgeon also had to carefully analyze the quality, position, vascularity, and mobility of the skin and muscle structures on both sides of the defect. The procedure began by elevating the skin flaps of the underlying abdominal musculature in a lateral direction towards the anterior axillary line. Next, the linea semilunaris was noted, along with the insertion of the external oblique aponeurosis (EOA). A vertically oriented incision located 2 cm laterally to Spigelian line and in the costal part of the external oblique aponeurosis, of 1 cm length, was made to identify and expand the plane beneath the EOA but superficial to the internal oblique fascia [2].
One must be careful not to dissect too deep into this layer to avoid injuring the internal oblique fascia or muscle. A deep dissection here may damage the segmental innervation of the rectus abdominis muscle or injure the Spigelian fascia, thus predisposing the patient to a Spigelian hernia. Generally, the planes are quite distinct. Once the plane is identified, the incision of the external oblique aponeurosis is extended cranially and caudally in its entire length. The dissection proceeds in this relatively avascular intermuscular plane and is continued in a lateral direction beyond the area of skin undermining to at least the level of the midaxillary line. At this point, the mobility of the innervated rectus abdominis-internal oblique abdominis-transversus abdominis muscle complex is determined.
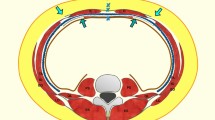
If additional mobility of these structures on either side of the midline is desired, then the dissection in the intermuscular plane can be continued to the posterior axillary line [3]. In our experience, each ipsilateral complex can be advanced towards the midline by 4 cm in the upper abdomen, 8 cm at the waist, and 3 cm in the lower abdomen. In the rare instances in which additional advancement is needed, the rectus muscle can be elevated off of the posterior rectus sheath in its entirety. Two centimeters of additional advancement can be obtained at each level by using this maneuver, and a mesh can reinforce the reconstruction, much like in a Rives-Stoppa repair [3]. The muscles are joined together in the midline with an interrupted or continuous closure using a strong non-absorbable suture (our preference is 0 polypropylene) (Fig. 1).
The skin flaps are then also advanced to the midline and approximated in a layered closure. Four suction drains are used routinely, and these are positioned in the plane between the oblique muscles and beneath the skin flaps on each side of the midline. They are brought out through separate stab incisions in the pubic area, lateral abdomen, or both. They are maintained until the drainage decreases to less than 30 cc per 24 h, which is usually at an average of 7 days.
When the peritoneal sac develops much further towards the lateral, for its release, the subcutaneous dissection reaches the vicinity of the linea semilunaris, or even exceeds it (Fig. 2, 3).
As an exception, if the length of the parietal defect is limited (supraumbilical, mesogastric, infraumbilical), the incision may be reduced, if it largely exceeds the defect [4]. If recreating the linea alba is possible after the unilateral practice of the EOA incision, contralateral anterior component release is not necessary (Fig. 4) [5]. The prosthesis is fixed in the onlay position and must cover the area of the component separation. To do this, the prosthesis is attached to the lateral edge of the EOA (Fig. 5).
Results
During the reference interval, the ACS procedure was used in 66 consecutive patients (41 men) with a mean age of 68.7 ± 8.5 years. All patients presented unique or associated comorbidities; obesity with a BMI over 32 kg/m2 was reported in 58 patients, diabetes in 38 patients, and COPD in 18 patients. Our indications included:
-
1.
recurrent incisional hernias with chronic infected mesh removal after incisional hernia repair in 28 patients. The onset of mesh infection was over 3 months after primary repair (mean time-lapse 19.8 months, range 3 months–25 years);
-
2.
open septic abdomen treated with negative-pressure wound therapy in 8 patients, open septic abdomen treated with Bogota bag in 10 patients. The main causes for open abdomen were anastomotic fistulas after colorectal surgery in 9 patients, after gastro-intestinal surgery in 3 patients, advanced primary peritonitis in 4 patients, and necrotic pancreatitis in the rest;
-
3.
abdominal primary or secondary tumor resection in 2 patients; and
-
4.
abdominal wall resection for multiple septic granulomas in 13 patients.
Of these, 29 patients (47%) presented postoperative complications. They were represented by skin necrosis in 13 patients, hematoma in 15 patients, and a burst abdomen in 1 patient. The mean hospital stay was 16.7 days (9–56 days); all wounds healed between 48 and 72 days (57.4 days). Recurrence was recorded in 18 patients (29%), all within the first year after the procedure. Of them, seven recurrences were noted in patients with postoperative hematoma, 1 after the burst abdomen, and the rest of them in patients without postoperative complications but with associated co-morbidities (5 in patients with BMI larger than 30 kg/m2, 3 in diabetic patients, and 2 in COPD patients). All recurrences were clinically and CT scan documented. All recurrent defects were located in the midline and were probably a healing or technical defect. There was no defect recorded lateral to Spiegel line. Only two of this patients were re-operated; defects smaller than 6 cm were primary closed and the suture was reinforced with a large sheet of light monofilament polypropylene 30 × 30 cm secured with cyanoacrylate blue.
-
5.
large incisional hernias with clean condition. We used MACS in five patient cases in which an extended subcutaneous supra-aponeurotic space was developed after sac dissection. In three patients, we practiced bilateral complete anterior component separation, and for the other 2 cases, unilateral limited anterior component separation. Patients were discharged after an average of 12.6 days (between 7 and 16 days). There were 2 patients with wound complications, represented by seroma, wound limited breakdown, and consecutive superficial infection. Wound healing was obtained after 58 and 94 days, respectively. Recurrence was seen in none of the five patients during postoperative follow-up sessions after at least one year.
Discussion
The closure of the abdominal wall defects using anterior components separation (ACS) was first reported by Albanese in the 50 s, but Ramirez et al. re-described and popularized it in 1990 [6, 7]. This technique is unique in that the functional transfer of the abdominal musculature provides stable and dynamic support to the abdominal wall, without the need for musculofascial flaps.
For 3 decades, ACS was the corner stone of abdominal wall reconstruction in patients with large incisional hernias. Mesh reinforcement, as on lay or underlay, was an important adjunct for improving outcomes due to its low recurrence rate. Many surgeons were reluctant to use it due to the logical sequelae of large myofascial and subcutaneous flap elevation. Some common complications are possible and these include seroma, hematoma, infection, skin edge necrosis, wound breakdown, and hernia recurrence [5, 8].
Although it has entered clinical use only in a few surgical services, in complex ventral hernia repairs, subcutaneous perfusion mapping with indocyanine green angiography in infrared light offers high sensitivity and facilitates the identification of ischemic tissues, thereby reducing the incidence of delayed wound healing and the risk of surgical site infection. This technique has the potential to reduce costs and improve patients’ quality of life by reducing the likelihood of wound complications and reoperation [9].
The complications resulting from ACS can be reduced using minimally invasive ACS, such as perforator sparing or endoscopic techniques [10, 11]. However, it should be noted that, in these procedures, the prosthesis will be placed in the sublay or underlay position, which increases the duration, complexity, and costs of the operation. Furthermore, in these technical variants, the prosthesis will not protect the weak area resulting from the EOA incision, increasing the risk of a lateral bulge or lateral abdominal wall hernia.
With regard to posterior component separation (PCS) and PCS-TAR, we believe it offers superior results to those otherwise obtained using MACS [5]. Most recent innovations have been achieved through the use of the minimally invasive approaches of endoscopic TAR (eTEP TAR) and robot-assisted TAR (rTAR) [8].
The information found in the literature related to incisional hernia is often contradictory. On the one hand, the onlay procedure retains its purpose and role [12, 13]; on the other hand, it is stated that MACS should be no longer performed because it has a high complication rate [14, 15]. Moreover, there are authors who view MACS and PCS-TAR as having comparable outcomes in complex abdominal wall reconstruction of midline ventral incisional hernias [10, 16].
To date, no comparative clinical or animal studies on the efficacy of various component techniques have been published. This is not surprising given that most surgeons prefer one technique over the other and the likelihood of a well-designed prospective randomized trial remains low [17, 18].
A challenging issue, for both patient and surgeon, is the recurrence after ACS without mesh because abdominal wall reconstruction is difficult to perform even for patients with small defects. Posterior component separation via TAR being it open or endoscopic is prohibitive in the first six months because of the important abdominal wall destabilization. After this period, the procedure can be considered with an in-lay mesh reinforcement but remains reserved only for specialized centers or dedicated surgeons with abdominal wall surgery expertize. On-lay mesh reinforcement could be a valid option in patients with small defects but the re-recurrence rate is not yet estimated.
For large midline hernia defects, if the release of the hernia sac requires extensive subcutaneous dissection, we consider the incision of the EOA to be a useful and acceptable option to close the midline. The morbidity of the operation does not derive from the EOA incision, but from the subcutaneous dissection, which has already been completed. If the dissection performed can allow for the installation of a sufficiently large mesh to reinforce the abdominal wall, we can proceed to practice MACS. In such situations, PSC-TAR or other technical procedures can be utilized to close the linea alba, although the morbidity following wide subcutaneous dissection will be added to that of wide retromuscular dissection.
MACS is not a complicated procedure compared to other component separations. For surgeons who frequently use onlay mesh placement, with the addition of EOA release, the proportion of cases in which it will be possible to close the medial defect will be higher than after the use of the classic relaxation incisions made in the anterior sheath of the rectus abdominis muscle.
We strongly believe that any hernia surgeon should be well versed in several techniques so they can offer the best technical option to patients with large incisional hernias.
Conclusion
Anterior component separation is still a valid option for reconstruction of the septic abdominal wall despite its high rate of wound complications. If a wide subcutaneous dissection is required, MACS remains a valid alternative in abdominal wall reconstruction in certain cases, but it is an exception due to the benefits of newly introduced posterior component separation via TAR.
Data Availability Statement
Data are available at request on the corresponding author.
References
Bower C, Roth SJ (2013) Economics of abdominal wall reconstruction. Surg Clin N Am 93:1241–1253
Lowe JB, Lowe Julie B, Baty JD, Garza JR (2003) Risks associated with “components separation” for closure of complex abdominal wall defects. Plast Reconstr Surg 111:1276–1285. https://doi.org/10.1097/01.PRS.00000472201.36879.FD
Shestak KC, Edington HJD, Johnson RR (2000) The separation of anatomic components technique for the reconstruction of massive midline abdominal wall defects: anatomy, surgical technique, applications and limitations revisited. Plast Reconstr Surg 105:731–739. https://doi.org/10.1097/00006534-200002000-00041
Punjani R, Shaikh I, Soni V (2015) Component separation technique: an effective way of treating large ventral hernia. Indian J Surg 77(3):S1476–S1479. https://doi.org/10.1007/s12262-015-1265-0
Thompson P, Losken A (2016) Open anterior component separation. In: Novitsky IW (ed) Hernia surgery. Current Principles, 1st edn. Springer International Publishing Switzerland, pp. 137–148
Albanese AR (1951) Eventración mediana xifoumbilical gigante: metodo para sutratamiento. Rev Asoc Med Arg 65:376
Ramirez OM, Ruas E, Dellon AL (1990) “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg 86(3):519–526. https://doi.org/10.1097/00006534-199009000-00023
Silverman RP (2012) Open component separation. In: Rosen MJ (ed) Atlas of Abdominal Wall Reconstruction, 1st edn. Elsevier, Philadelphia, pp 130–138
Colavita PD, Wormer BA, Belyansky I, Lincourt A, Getz SB, Heniford BT, Augenstein VA (2016) Intraoperative indocyanine green fluorescence angiography to predict wound complications in complex ventral hernia repair. Hernia 20:139–149. https://doi.org/10.1007/s00421-008-0955-8
Hodgkinson JD, Leo CA, Maeda Y, Bassett P, Oke SM, Vaizey CJ, Warusavitarne J (2018) A metaanalysis comparing open anterior component separation with posterior component separation and transversus abdominis release in the repair of midline ventral hernias. Hernia 22:617–626. https://doi.org/10.1007/s10029-018-1757-5
Holihan JL, Askenasy EP, Greenberg JA, Keith JN, Martindale RG, Roth JS, Mo J, Ko TC, Kao LS, Liang MK (2016) Component separation vs bridged repair for large ventral hernias a multi-institutional risk-adjusted comparison, systematic review, and meta-analysis. Surg Infect (Larchmt) 17(1):17–26. https://doi.org/10.1089/sur.2015.124
Holihan JL, Nguyen DH, Nguyen MT, Mo J, Kao LS, Liang MK (2016) mesh location in open ventral hernia repair: a systematic review and network meta-analysis. World J Surg 40:89–99. https://doi.org/10.1007/s00268-015-3252-9
Köckerling K (2018) Onlay technique in incisional hernia repair – a systematic review. Front Surg 5:71. https://doi.org/10.3389/fsurg.2018.00071
Scheuerlein H, Thiessen A, Schug-Pass C, Köckerling F (2018) What do we know about component separation techniques for abdominal wall hernia repair? Front Surg 5:24. https://doi.org/10.3389/fsurg.2018.00024
de VriesReilingh TS, van Goor H, Charbon JA (2007) Repair of giant midline abdominal wall hernias: “components separation technique” versus prosthetic repair. World J Surg 31:756–763. https://doi.org/10.1007/s00268-006-0502-x
Maloney SR, Schlosser KA, Prasad T, Colavita PD, Kercher KW, Augenstein VA, Heniford BT (2020) The impact of component separation technique versus no component separation technique on complications and quality of life in the repair of large ventral hernias. Surg Endosc 34:981–987. https://doi.org/10.1007/s00464-019-06892-x
Novitsky YW, Belyansky I (2018) Discussion: anterior versus posterior component separation: which is better? Plast Reconstr Surg 142(3):56S-57S. https://doi.org/10.1097/PRS.0000000000004880
Oprea V, Radu VG, Moga D (2016) Transversus abdominis muscle release (TAR) for large incisional hernia repair. Chirurgia 111:535–540. https://doi.org/10.21614/chirurgia.111.6.535
Author information
Authors and Affiliations
Contributions
Doru Moga and Valentin Oprea wrote the article, which all authors critically revised. All authors agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare competing interests.
Guarantor
Doru Moga.
Valentin Oprea.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moga, D., Oprea, V. Open Anterior Component Separation for Complex Incisional and Ventral Hernias—When and How? Case Series Analysis. Indian J Surg (2022). https://doi.org/10.1007/s12262-022-03516-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12262-022-03516-y