Abstract
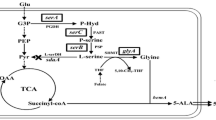
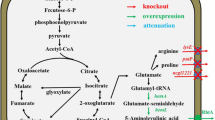
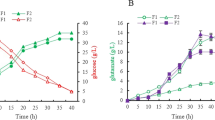
5-Aminolevulinic acid (ALA), a valuable nonproteinogenic amino acid, has received increasing attention in various fields including medicine, agriculture, and cosmetics. Here, we developed metabolically engineered Corynebacterium glutamicum to enhance ALA production. To achieve this object, we focused on the flux redistribution of the TCA cycle toward l-glutamate and introduction of the heterogenous ALA transporter in C. glutamicum. First, the oxoglutarate dehydrogenase inhibitor (OdhI) was mutated by site-directed mutagenesis to prevent the phosphorylation that abolishes the capability of OdhI protein to inhibit oxoglutarate dehydrogenase complex activity. The overexpression of the double-mutated OdhI, T14A/T15A, showed the highest l-glutamate and ALA production compared with that of the native and single-mutated OdhI. To increase ALA secretion from the engineered strain, the ALA exporter RhtA from Escherichia coli was introduced and allowed 2.46 ± 0.11 g/L of ALA production, representing a 1.28-fold increase in extracellular ALA production. In the final strain, the induction of triggers, including Tween 40 and ethambutol, was performed to amplify the effect of the flux redistribution toward ALA. A significant increase in ALA production was observed in the induction of triggers. In particular, ethambutol induction showed the best result, corresponding to 2.9 ± 0.15 g/L of ALA production. Therefore, this biotechnological model enables the efficient extracellular production of ALA from glucose in C. glutamicum.
Similar content being viewed by others
References
Liu, L., J. L. Martinez, Z. Liu, D. Petranovic, and J. Nielsen (2014) Balanced globin protein expression and heme biosynthesis improve production of human hemoglobin in Saccharomyces cerevisiae. Metab. Eng. 21: 9–16.
Moore, S. J., A. D. Lawrence, R. Biedendieck, E. Deery, S. Frank, M. J. Howard, S. E. J. Rigby, and M. J. Warren (2013) Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B-12). Proc. Natl. Acad. Sci. USA. 110: 14906–14911.
Yu, C. H. and C. C. Yu (2014) Photodynamic therapy with 5-aminolevulinic acid (ALA) impairs tumor initiating and chemoresistance property in head and neck cancer-derived cancer stem cells. PLoS One. 9: e87129.
Nguyen, H., H. S. Kim, and S. Jung (2016) Altered tetrapyrrole metabolism and transcriptome during growth-promoting actions in rice plants treated with 5-aminolevulinic acid. Plant Growth Regul. 78: 133–144.
Tran, N. T., D. N. Pham, and C. J. Kim (2019) Production of 5-aminolevulinic acid by recombinant Streptomyces coelicolor expressing hemA from Rhodobacter sphaeroides. Biotechnol. Bioprocess Eng. 24: 488–499.
Ramzi, A. B., J. E. Hyeon, S. W. Kim, C. Park, and S. O. Han (2015) 5-Aminolevulinic acid production in engineered Corynebacterium glutamicum via C5 biosynthesis pathway. Enzyme Microb. Technol. 81: 1–7.
Sasaki, K., M. Watanabe, T. Tanaka, and T. Tanaka (2002) Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl. Microbiol. Biotechnol. 58: 23–29.
Zhang, J., H. Weng, Z. Zhou, G. Du, and Z. Kang (2019) Engineering of multiple modular pathways for high-yield production of 5-aminolevulinic acid in Escherichia coli. Bioresour. Technol. 274: 353–360.
Xie, L., M. A. Eiteman, and E. Altman (2003) Production of 5-aminolevulinic acid by an Escherichia coli aminolevulinate dehydratase mutant that overproduces Rhodobacter sphaeroides aminolevulinate synthase. Biotechnol. Lett. 25: 1751–1755.
Fu, W., J. Lin, and P. Cen (2007) 5-aminolevulinate production with recombinant Escherichia coli using a rare codon optimizer host strain. Appl. Microbiol. Biotechnol. 75: 777–782.
Yang, P., W. Liu, X. Cheng, J. Wang, Q. Wang, and Q. Qi (2016) A new strategy for production of 5-aminolevulinic acid in recombinant Corynebacterium glutamicum with high yield. Appl. Environ. Microbiol. 82: 2709–2717.
Feng, L., Y. Zhang, J. Fu, Y. Mao, T. Chen, X. Zhao, and Z. Wang (2016) Metabolic engineering of Corynebacterium glutamicum for efficient production of 5-aminolevulinic acid. Biotechnol. Bioeng. 113: 1284–1293.
Ding, W., H. Weng, G. Du, J. Chen, and Z. Kang (2017) 5-Aminolevulinic acid production from inexpensive glucose by engineering the C4 pathway in Escherichia coli. J. Ind. Microbiol. Biotechnol. 44: 1127–1135.
Wang, L., S. Wilson, and T. Elliott (1999) A mutant HemA protein with positive charge close to the N terminus is stabilized against heme-regulated proteolysis in Salmonella typhimurium. J. Bacteriol. 181: 6033–6041.
Kang, Z., Y. Wang, P. Gu, Q. Wang, and Q. Qi (2011) Engineering Escherichia coli for efficient production of 5-aminolevulinic acid from glucose. Metab. Eng. 13: 492–498.
Zhang, J., Z. Kang, J. Chen, and G. Du (2015) Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli. Sci. Rep. 5: 8584.
Noh, M. H., H. G. Lim, S. Park, S. W. Seo, and G. Y. Jung (2017) Precise flux redistribution to glyoxylate cycle for 5-aminolevulinic acid production in Escherichia coli. Metab. Eng. 43: 1–8.
Shirai, T., K. Fujimura, C. Furusawa, K. Nagahisa, S. Shioya, and H. Shimizu (2007) Study on roles of anaplerotic pathways in glutamate overproduction of Corynebacterium glutamicum by metabolic flux analysis. Microb. Cell Fact. 6: 19.
Kim, J., T. Hirasawa, M. Saito, C. Furusawa, and H. Shimizu (2011) Investigation of phosphorylation status of OdhI protein during penicillin- and Tween 40-triggered glutamate overproduction by Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 91: 143–151.
Wen, J. and J. Bao (2019) Engineering Corynebacterium glutamicum triggers glutamic acid accumulation in biotin-rich corn stover hydrolysate. Biotechnol. Biofuels. 12: 86.
Joo, Y. C., S. K. You, S. K. Shin, Y. J. Ko, K. H. Jung, S. A. Sim, and S. O. Han (2017) Bio-based production of dimethyl itaconate from rice wine waste-derived itaconic acid. Biotechnol. J. 12: 1700114.
Joo, Y. C., Y. J. Ko, S. K. You, S. K. Shin, J. E. Hyeon, A. S. Musaad, and S. O. Han (2018) Creating a new pathway in Corynebacterium glutamicum for the production of taurine as a food additive. J. Agric. Food Chem. 66: 13454–13463.
Jeong, Y. J., J. W. Choi, M. S. Cho, and K. J. Jeong (2019) Isolation of novel exo-type β-agarase from Gilvimarinus chinensis and high-level secretory production in Corynebacterium glutamicum. Biotechnol. Bioprocess Eng. 24: 250–257.
Xu, D. Y., J. Zhao, G. Cao, J. Wang, Q. Li, P. Zheng, S. Zhao, and J. Sun (2018) Removal of feedback inhibition of Corynebacterium glutamicum phosphoenolpyruvate carboxylase by addition of a short terminal peptide. Biotechnol. Bioprocess Eng. 23: 72–78.
Yu, X., H. Jin, W. Liu, Q. Wang, and Q. Qi (2015) Engineering Corynebacterium glutamicum to produce 5-aminolevulinic acid from glucose. Microb. Cell Fact. 14: 183.
Zhang, B. and B. C. Ye (2018) Pathway engineering in Corynebacterium glutamicum S9114 for 5-aminolevulinic acid production. 3 Biotech. 8: 247.
Asakura, Y., E. Kimura, Y. Usuda, Y. Kawahara, K. Matsui, T. Osumi, and T. Nakamatsu (2007) Altered metabolic flux due to deletion of odhA causes L-glutamate overproduction in Corynebacterium glutamicum. Appl. Environ. Microbiol. 73: 1308–1319.
Niebisch, A., A. Kabus, C. Schultz, B. Weil, and M. Bott (2006) Corynebacterial protein kinase G controls 2-oxoglutarate dehydrogenase activity via the phosphorylation status of the OdhI protein. J. Biol. Chem. 281: 12300–12307.
Kim, J., H. Fukuda, T. Hirasawa, K. Nagahisa, K. Nagai, M. Wachi, and H. Shimizu (2010) Requirement of de novo synthesis of the OdhI protein in penicillin-induced glutamate production by Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 86: 911–920.
Nara, T., H. Samejima, and S. Kinoshita (1964) Effect of penicillin on amino acid fermentation. Agric. Biol. Chem. 28: 120–124.
Takinami, K., H. Yoshii, H. Tsuri, and H. Okada (1965) Biochemical effects of fatty acid and its derivatives on L-glutamic acid fermentation Part 3. Biotin-tween 60 relationship in accumulation of L-glutamic acid and growth of Brevibacterium Lactofermentum. Agric. Biol. Chem. 29: 351–359.
Radmacher, E., K. C. Stansen, G. S. Besra, L. J. Alderwick, W. N. Maughan, G. Hollweg, H. Sahm, V. F. Wendisch, and L. Eggeling (2005) Ethambutol, a cell wall inhibitor of Mycobacterium tuberculosis, elicits L-glutamate efflux of Corynebacterium glutamicum. Microbiology. 151: 1359–1368.
Shiio, I., S. I. Otsuka, and N. Katsuya (1962) Effect of biotin on the bacterial formation of glutamic acid. II. Metabolism of glucose. J. Biochem. 52: 108–116.
Eikmanns, B. J., E. Kleinertz, W. Liebl, and H. Sahm (1991) A family of Corynebacterium glutamicum/Escherichia coli shuttle vectors for cloning, controlled gene expression, and promoter probing. Gene. 102: 93–98.
Jiang, Y., F. Qian, J. Yang, Y. Liu, F. Dong, C. Xu, B. Sun, B. Chen, X. Xu, Y. Li, R. Wang, and S. Yang (2017) CRISPR-Cpf1 assisted genome editing of Corynebacterium glutamicum. Nat. Commun. 8: 15179.
Cleto, S., J. V. Jensen, V. F. Wendisch, and T. K. Lu (2016) Corynebacterium glutamicum metabolic engineering with CRISPR interference (CRISPRi). ACS Synth. Biol. 5: 375–385.
Ko, Y. J., Y. C. Joo, J. E. Hyeon, E. Lee, M. E. Lee, J. Seok, S. W. Kim, C. Park, and S. O. Han (2018) Biosynthesis of organic photosensitizer Zn-porphyrin by diphtheria toxin repressor (DtxR)-mediated global upregulation of engineered heme biosynthesis pathway in Corynebacterium glutamicum. Sci. Rep. 8: 14460.
van der Rest, M. E., C. Lange, and D. Molenaar (1999) A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl. Microbiol. Biotechnol. 52: 541–545.
Park, S. H., H. U. Kim, T. Y. Kim, J. S. Park, S. S. Kim, and S. Y. Lee (2014) Metabolic engineering of Corynebacterium glutamicum for L-arginine production. Nat. Commun. 5: 4618.
Mauzerall, D. and S. Granick (1956) The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J. Biol. Chem. 219: 435–446.
Schultz, C., A. Niebisch, L. Gebel, and M. Bott (2007) Glutamate production by Corynebacterium glutamicum: dependence on the oxoglutarate dehydrogenase inhibitor protein OdhI and protein kinase PknG. Appl. Microbiol. Biotechnol. 76: 691–700.
Nguyen, A. Q. D., J. Schneider, G. K. Reddy, and V. F. Wendisch (2015) Fermentative production of the diamine putrescine: System metabolic engineering of Corynebacterium Glutamicum. Metabolites. 5: 211–231.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2018R1A2B2003704) and supported by a Korea University Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ko, Y.J., You, S.K., Kim, M. et al. Enhanced Production of 5-aminolevulinic Acid via Flux Redistribution of TCA Cycle toward l-Glutamate in Corynebacterium glutamicum. Biotechnol Bioproc E 24, 915–923 (2019). https://doi.org/10.1007/s12257-019-0376-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0376-z