Abstract
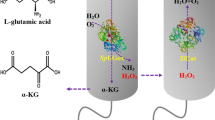
L-amino acid oxidase (AAO) was reported to be capable of converting L-glutamic acid to α-aketoglutaric acid (α-KG). The sequence of AAO from Kitasatospora cheerisanensis was synthesized based on Pichia pastoris codon-usage preferences. AAO gene was cloned into plasmid pPICZα which was transformed into P. pastoris. Next, multi-copy expression plasmids were constructed by using plasmid pHBM905BDM. High-density fermentation was performed and the recombinant enzyme was characterized. The conversion conditions were optimized. By using Escherichia coli expression system, no soluble or active AAO was obtained from two strains after fermentation and induction. We can’t obtain high-level expression of recombinant strains by using plasmid pPICZα. Therefore, we constructed multi-copy expression plasmids using plasmid pHBM905BDM. By using this plasmid, multi-copy strains were constructed and named as PAAO1, PAAO2, PAAO3, PAAO4, and PAAO5, respectively. The following results showed that expression of AAO in multicopy strains increased as designed and strain PAAO5 was chosen for high-density fermentation and enzyme activity experiments. After high-density fermentation, we achieved an AAO-expression yield of 120.8 U/mL. After temperature and pH optimization, the highest AAO activity was observed at a temperature and pH of 20°C and 6, respectively. After optimization of the conversion conditions, the average production rate of L-glutamic acid to α-KG was 3.46 g/L/h and the highest α-KG titer (103 g/L) was converted from 120 g/L L-glutamic acid. In this study, AAO was abundantly expressed by using P. pastoris expression system. The following experiments indicated that AAO is suitable for use in industrial applications.
Similar content being viewed by others
Availability of Data and Materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- AAO:
-
L-amino acid oxidase
- α-KG:
-
α-aketoglutaric acid
References
Doucette, C. D., D. J. Schwab, N. S. Wingreen, and J. D. Rabinowitz (2011) α-ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition. Nat. Chem. Biol. 7: 894–901.
Morgunov, I. G., S. V. Kamzolova, and V. A. Samoilenko (2013) Enhanced α-ketoglutaric acid production and recovery in Yarrowia lipolytica yeast by effective pH controlling. Appl. Microbiol. Biotechnol. 97: 8711–8718.
Finogenova, T. V., I. G. Morgunov, S. V. Kamzolova, and O. G. Chernyavskaya (2005) Organic acid production by the yeast Yarrowia lipolytica: a review of prospects. Appl. Biochem. Microbiol. 41: 418–425.
Stottmeister, U., A. Aurich, H. Wilde, J. Andersch, S. Schmidt, and D. Sicker (2005) White biotechnology for green chemistry: fermentative 2-oxocarboxylic acids as novel building blocks for subsequent chemical syntheses. J. Ind. Microbiol. Biotechnol. 32: 651–664.
Otto, C., V. Yovkova, and G. Barth (2011) Overproduction and secretion of alphaketoglutaric acid by microorganisms. Appl. Microbiol. Biotechnol. 92: 689–695.
Yovkova, V., C. Otto, A. Aurich, S. Mauersberger, and G. Barth (2014) Engineering the α-ketoglutarate overproduction from raw glycerol by overexpression of the genes encoding NADH+-dependent isocitrate dehydrogenase and pyruvate carboxylase in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 98: 2003–2013.
Liu, L., Y. Li, Y. Zhu, G. Du, and J. Chen (2007) Redistribution of carbon flux in Torulopsis glabrata by altering vitamin and calcium level. Metab. Eng. 9: 21–29.
Yu, Z., G Du, J. Zhou, and J. Chen (2012) Enhanced alpha-ketoglutaric acid production in Yarrowia lipolytica WSH-Z06 by an improved integrated fed-batch strategy. Bioresour. Technol. 114: 597–602.
Falcioni, F., L. M. Blank, O. Frick, A. Karau, B. Bühler, and A. Schmid (2009) Proline availability regulates proline-4-hydroxylase synthesis and substrate uptake in proline-hydroxylating recombinant Escherichia coli. Appl. Environ. Microbiol. 79: 3091–3100.
Liu, L., G. S. Hossain, H. D. Shin, J. Li, G. Du, and J. Chen (2013) One-step production of alpha-ketoglutaric acid from glutamic acid with an engineered l-amino acid deaminase from Proteus mirabilis. J. Biotechnol. 164: 97–104.
Bornscheuer, U. T., G. W. Huisman, R. J. Kazlauskas, S. Lutz, J. C. Moore, and K. Robins (2012) Engineering the third wave of biocatalysis. Nature. 485: 185–194.
YaPing, W., R. Ben, Y. Hong, H. Rui, L. Li, L. Ping’an, and M. Lixin (2017) High-level expression of L -glutamate oxidase in Pichia pastoris using multi-copy expression strains and high cell density cultivation. Protein. Expr. Purif. 129: 108–114.
Fan, X., R. Chen, L. Chen, and L. Liu (2016) Enhancement of alpha-ketoglutaric acid production from l-glutamic acid by high-cell-density cultivation. J. Mol. Catal. B Enzym. 126: 10–17.
Niu, P., X. Dong, Y. Wang, and L. Liu (2014) Enzymatic production of α-ketoglutaric acid from l-glutamic acid via l-glutamate oxidase. J. Biotechnol. 179: 56–62.
Yang, H., C. Zhai, X. Yu, Z. Li, W. Tang, Y. Liu, X. Ma, X. Zhong, G. Li, D. Wu, and L. Ma (2016) High-level expression of Proteinase K from Tritirachium album Limber in Pichia pastoris using multi-copy expression strains. Protein. Expr. Purif. 122: 38–44.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227: 680–685.
Allain, C. C., L. S. Poon, C. S. Chan, W. Richmond, and P. C. Fu (1974) Enzymatic determination of total serum cholesterol. Clin. Chem. 20: 470–475.
Zhang, D., N. Liang, Z. Shi, L. Liu, J. Chen, and G. Du (2009) Enhancement of α-ketoglutarate production in Torulopsis glabrata: redistribution of carbon flux from pyruvate to -ketoglutarate. Biotechnol. Bioprocess Eng. 14: 134–139.
Kommoju, P. R., P. Macheroux, and S. Ghisla (2007) Molecular cloning, expression and purification of l-amino acid oxidase from the Malayan pit viper Calloselasma rhodostoma. Protein. Expr. Purif. 52: 89–95.
Acknowledgments
We acknowledge financial support of National Key R D Program of China (2017YFD0200900) Subject 2 (2017YFD0200902), Joint Open Fund of National Biopesticide Engineering Research Centre and Scientific Observation and Experimental Station of Utilization of microbial resources (Central China), Ministry of Agriculture and Rural Affairs (Grant No. JF-NBCOES-1807), Natural Science Foundation of China (31300074), Natural Science Foundation of Hubei Province (2014CFB541), Specialized Research Fund for the Doctoral Program of Higher Education (20124208120004), Key project of educational commission of Hubei province of China (D20171002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations Ethics statement and consent to participate and Not applicable.
Competing Interests The authors declare that they have no competing interests.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ben, R., Xianqing, L., Fang, L. et al. Expression and Characterization of a New L-amino Acid Oxidase AAO Producing α-ketoglutaric Acid from L-glutamic Acid. Biotechnol Bioproc E 24, 981–989 (2019). https://doi.org/10.1007/s12257-019-0182-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0182-7