Abstract
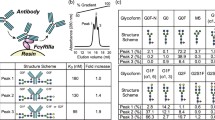
Site specific glycosylation of immunoglobulin G (IgG) occurs at Asn297 in the Fc region. The heterogeneous ensemble of glycoform occurs due to the degree of terminal galactosylation and sialylation, and these differences in glycosylation affect both the pharmacokinetic behavior and effector functions of the IgG, such as complementdependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC). In this study, the differential glycosylation of IgG was compared and environmental physical and chemical parameters were evaluated in an attempt to promote glycosylation of recombinant antibodies, thereby creating more humanized glycoform antibodies and increasing their in vivo efficacy as therapeutic drugs. It was shown that cells at late stationary growth phase in batch cultures, cells with increased passage number, and the culture conditions of lowered temperature and pH promoted galactosylation and sialylation of antibodies. Galactose, fructose and mannose were found to elicit galactosylation and sialylation when they were used alone as a substitute of glucose. Mannose showed synergistic effects on glycosylation when used with other sugars, such as glucose and galactose. However when fructose was used with other sugars, the degree of galactosylation mechanism appeared to be decreased. These results support understandings of the glycosylation mechanisms in glycoprotein, particularly recombinant antibodies for therapeutics.
Similar content being viewed by others
References
Burton, D. R. and R. A. Dwek (2006) Immunology. Sugar determines antibody activity. Scienc. 313: 670–673.
Raju, T. S., J. B. Briggs, S. M. Borge, and A. J. Jones (2000) Species–specific variation in glycosylation of IgG: evidence for the species–specific sialylation and branch–specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiolog. 10: 477–486.
Raju, T. S., J. B. Briggs, S. M. Chamow, M. E. Winkler, and A. J. Jones (2001) Glycoengineering of therapeutic glycoproteins: in vitro galactosylation and sialylation of glycoproteins with terminal N–acetylglucosamine and galactose residues. Biochemistr. 40: 8868–8876.
Sha, S., C. Agarabi, K. Brorson, D. Y. Lee, and S. Yoon (2016) N–Glycosylation design and control of therapeutic monoclonal antibodies. Trends Biotechnol. 34: 835–846.
Nimmerjahn, F., R. M. Anthony, and J. V. Ravetch (2007) Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl. Acad. Sci. US. 104: 8433–8437.
Hodoniczky, J., Y. Z. Zheng, and D. C. James (2005) Control of recombinant monoclonal antibody effector functions by Fc Nglycan remodeling in vitro. Biotechnol. Prog. 21: 1644–1652.
Scallon, B. J., S. H. Tam, S. G. McCarthy, A. N. Cai, and T. S. Raju (2007) Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol. Immunol. 44: 1524–1534.
Parekh, R., D. Isenberg, G. Rook, I. Roitt, R. Dwek, and T. A. Rademacher (1989) Comparative analysis of disease–associated changes in the galactosyl ation of serum IgG. J. Autoimmun. 2: 101–114.
Dwek, R. A., A. C. Lellouch, and M. R. Wormald (1995) Glycobiology: ‘The function of sugar in the IgG molecule’. J. Anat. 187: 279–292.
Gindzienska–Sieskiewicz, E., P. A. Klimiuk, D. G. Kisiel, A. Gindzienski, and S. Sierakowski (2007) The changes in monosaccharide composition of immunoglobulin G in the course of rheumatoid arthritis. Clin Rheumatol. 26: 685–690.
Kaneko, Y., F. Nimmerjahn, and J. V. Ravetch (2006) Antiinflammatory activity of immunoglobulin G resulting from Fc sialylation. Scienc. 313: 627–628.
Krapp, S., Y. Mimura, R. Jefferis, R. Huber, and P. Sondermann (2003) Structural analysis of human IgG–Fc glycoforms reveals a correlation between glycosylation and structural integrity. J. Mol. Biol. 325: 979–989.
Malhotra, R., M. R. Wormald, P. M. Rudd, P. B. Fischer, R. A. Dwek, and R. B. Sim (1995) Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose–binding protein. Nat. Med. 1:237–243.
Matsumoto, A., K. Shikata, F. Takeuchi, N. Kojima, and T. Mizuochi (2000) Autoantibody activity of IgG rheumatoid factor increases with decreasing levels of galactosylation and sialylation. J. Biochem. 128: 621–628
Novak, J., M. Tomana, G. R. Shah, R. Brown, and J. Mestecky (2005) Heterogeneity of IgG glycosylation in adult periodontal disease. J. Dent. Res. 84: 897–901.
Li, H., N. Sethuraman, T. A. Stadheim, D. Zha, B. Prinz, N. Ballew, P. Bobrowicz, B. K. Choi, W. J. Cook, M. Cukan, N. R. Houston–Cummings, R. Davidson, B. Gong, S. R. Hamilton, J. P. Hoopes, Y. Jiang, N. Kim, R. Mansfield, J. H. Nett, S. Rios, R. Strawbridge, S. Wildt, and T. U. Gerngross (2006) Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat. Biotechnol. 24: 210–215.
Routier, F. H., M. J. Davies, K. Bergemann, and E. F. Hounsell (1997) The glycosylation pattern of humanized IgGI antibody (D1.3) expressed in CHO cells. Glycoconj J. 14: 201–207.
Baker, K. N., M. H. Rendall, A. E. Hills, M. Hoare, P. B. Freedman, and D. C. James (2001) Metabolic control of recombinant protein N–glycan processing in NS0 and CHO cells. Biotechnol. Bioeng. 73: 188–202.
Leist, C. H., H. P. Meyer, and A. Fiechter (1990) Potential and problems of animal cells in suspension culture. J. Biotechnol. 15: 1–46.
Lifely, M. R., C. Hale, S. Boyce, M. J. Keen, and J. Phillips (1995) Glycosylation and biological activity of CAMPATH–1H expressed in different cell lines and grown under different culture conditions. Glycobiolog. 5: 813–822.
Fogolin, M. B., R. Wagner, M. Etcheverrigaray, and R. Kratje (2004) Impact of temperature reduction and expression of yeast pyruvate carboxylase on hGM–CSF–producing CHO cells. J. Biotechnol. 109: 179–191.
Furukawa, K. and K. Ohsuye (1998) Effect of culture temperature on a recombinant CHO cell line producing a Cterminal alpha–amidating enzyme. Cytotechnolog. 26: 153–164.
Trummer, E., K. Fauland, S. Seidinger, K. Schriebl, C. Lattenmayer, R. Kunert, K. Vorauer–Uhl, R. Weik, N. Borth, H. Katinger, and D. Müller (2006) Process parameter shifting: PartI. Effect of DOT, pH, and temperature on the performance of Epo–Fc expressing CHO cells cultivated in controlled batch bioreactors. Biotechnol. Bioeng. 94: 1033–1044.
Yoon, S. K., S. L. Choi, J. Y. Song, and G. M. Lee (2005) Effect of culture pH on erythropoietin production by Chinese hamster ovary cells grown in suspension at 32.5 and 37.0 degrees. C. Biotechnol. Bioeng. 89: 345–356.
Ahn, W. S., J. J. Jeon, Y. R. Jeong, S. J. Lee, and S. K. Yoon (2008) Effect of culture temperature on erythropoietin production and glycosylation in a perfusion culture of recombinant CHO cells. Biotechnol. Bioeng. 101: 1234–1244.
Nam, J. H., F. Zhang, M. Ermonval, R. J. Linhardt, and S. T. Sharfstein (2008) The effects of culture conditions on the glycosylation of secreted human placental alkaline phosphatase produced in Chinese hamster ovary cells. Biotechnol. Bioeng. 100: 1178–1192.
Hossler, P., S. F. Khattak, and Z. J. Li (2009) Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiolog. 19: 936–949.
Gawlitzek, M., U. Valley, and R. Wagner (1998) Ammonium ion and glucosamine dependent increases of oligosaccharide complexity in recombinant glycoproteins secreted from cultivated BHK–21 cells. Biotechnol. Bioeng. 57: 518–528.
Gawlitzek, M., T. Ryll, J. Lofgren, and M. B. Sliwkowski (2000) Ammonium alters N–glycan structures of recombinant TNFRIgG: degradative versus biosynthetic mechanisms. Biotechnol. Bioeng. 68: 637–646.
Altamirano, C., C. Paredes, A. Illanes, J. J. Cairó, and F. Gòdia (2004) Strategies for fed–batch cultivation of t–PA producing CHO cells: substitution of glucose and glutamine and rational design of culture medium. J. Biotechnol. 110: 171–179.
Swinnen, K., A. Krul, I. Van Goidsenhoven, N. Van Tichelt, A. Roosen, and K. Van Houdt (2007) Performance comparison of protein A affinity resins for the purification of monoclonal antibodies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 848: 97–107.
Tarentino, A. L. and T. H. Plummer Jr. (1994) Enzymatic deglycosylation of asparagine–linked glycans: purification, properties, and specificity of oligosaccharide–cleaving enzymes from Flavobacterium meningosepti cum. Methods Enzymol. 230: 44–57.
Anumula, K. R. and P. Du (1999) Characterization of carbohydrates using highly fluorescent 2–aminobenzoic acid tag following gel electrophoresis of glycoproteins. Anal. Biochem. 275: 236–242.
Dhume, S. T., G. N. Saddic, and K. R Anumula (2008) Monitoring glycosylation of therapeutic glycoproteins for consistency by HPLC using highly fluorescent anthranilic acid (AA) Tag. Methods Mol. Biol. 446: 317–331.
Anumula, K. R. and S. T. Dhume (1998) High resolution and high sensitivity methods for oligosaccharide mapping and characterization by normal phase high performance liquid chromatography following derivatization with highly fluorescent anthranilic acid. Glycobiolog. 8: 685–694.
Anumula, K. R. (2006) Advances in fluorescence derivatization methods for high–performance liquid chromatographic analysis of glycoprotein carbohydrates. Anal. Biochem. 350: 1–23.
Aknowledgement
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015M3A9135053958).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SM., Chang, KH. & Oh, D.J. Effect of Environmental Parameters on Glycosylation of Recombinant Immunoglobulin G Produced from Recombinant CHO Cells. Biotechnol Bioproc E 23, 456–464 (2018). https://doi.org/10.1007/s12257-018-0109-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-018-0109-8