Abstract
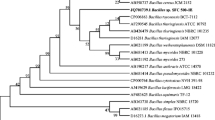
The objective of this study was to isolate a new bacterium and investigate its ability for degradation of various toxic organic compounds. Based on 16S rRNA gene sequence and phylogenetic analysis, the isolated strain was identified as Bacillus sp. CYR2. Degradation of various toxic compounds and growth of CYR2 strain were evaluated with 2 and 4% inoculum sizes. All the experiments were conducted for 6 days, flasks were incubated at 30oC under 180 rpm. Among the 2 and 4% inoculum sizes, bacteria showed highest growth and toxic compounds degradation at 4% inoculum size. Especially, compared to 2% inoculum size, growth of the strain CYR2 at 4% inoculum size was increased by 15.1 folds with 4-secondarybutylphenol, 9.1 folds with phenol, and 5.4 folds with 4-tertiary-butylphenol. Strain CYR2 at 4% inoculum size showed highest removal of phenol (84 ± 5%), followed by 4-tertiary-butylphenol (66 ± 3%), 4-secondary-butylphenol (63 ± 5%) and 4-nonylphenol (57 ± 6%). Compared with 2% inoculum size, degradation ability of strain CYR2 with 4% inoculum size was enhanced by 3.45 times with 4-tertiary-octylphenol, and 2.53 times with 4-tertiarybutylphenol. Our results indicated that the newly isolated Bacillus sp. CYR2 can be used for in situ bioremediation of phenol and alkylphenols contaminated water.
Similar content being viewed by others
References
Chang, Y. C., K. Takada, D. B. Choi, T. Toyama, K. Sawada, and S. Kikuchi (2013) Isolation of biphenyl and polychlorinated biphenyl-degrading bacteria and their degradation pathway. Appl. Biochem. Biotechnol. 170: 381–398.
Harzallah, B., H. Bousseboua, and Y. Jouanneau (2017) Diversity shift in bacterial phenol hydroxylases driven by alkyl-phenols in oil refinery wastewaters. Environ. Sci. Pollut. Res. DOI 10.1007/s11356-017-8950-4.
Das, B., T. K. Mandal, and S. Patra (2016) Biodegradation of phenol by a novel diatom BD1IITG-kinetics and biochemical studies. Intern. J. Environ. Sci. Technol. 13: 529–542.
Liu, D., J. Liu, M. Guo, H. Xu, S. Zhang, L. Shi, and C. Yao (2016) Occurrence, distribution, and risk assessment of alkylphenols, bisphenol A, and tetrabromobisphenol A in surface water, suspended particulate matter, and sediment in Taihu Lake and its tributaries. Mar. Poll. Bull. 112: 142–150.
Ros, A., A. Vallejo, M. Olivares, N. Etxebarria, and A. Prieto (2016) Determination of endocrine disrupting compounds in fish liver, brain, and muscle using focused ultrasound solid–liquid extraction and dispersive solid phase extraction as clean-up strategy. Anal. Bioanal. Chem. 408: 5689–5700.
Belkhamssa, N., J. P. da Costa, C. I. L. Justino, P. S. M. Santos, S. Cardoso, A. C. Duarte, T. R. Santos, and M. Ksibi (2016) Development of an electrochemical biosensor for alkylphenol detection. Talanta 158: 30–34.
Lofthus, S., I. K. Almas, P. Evans, O. Pelz, and O. G. Brakstad (2016) Biotransformation of potentially persistent alkylphenols in natural seawater. Chemosp. 156: 191–194.
Jia, Y., S. Hao, Y. L. E. Wong, X. Chen, and T. W. D. Chan (2016) Thermo-responsive polymer tethered metal-organic framework core-shell magnetic microspheres for magnetic solidphase extraction of alkylphenols from environmental water samples. J. Chromatogra A. 1456: 42–48.
Duan, X. Y., Y. Li, X. Li, D. Zhang, and Y. Gao (2014) Alkylphenols in surface sediments of the Yellow Sea and East China Sea inner shelf: Occurrence, distribution and fate. Chemosp. 107: 265–273.
Hanioka, N., T. Isobe, S. Ohkawara, T. T. Kagawa, and H. Jinno (2017) Glucuronidation of 4-tert-octylphenol in humans, monkeys, rats, and mice: An in vitro analysis using liver and intestine microsomes. Arch. Toxicol. 91: 1–6.
Takeo M., S. K. Prabu, C. Kitamura, M. Hirai, H. Takahashi, D. Kato, and S. Negoro (2006) Characterization of alkylphenol degradation gene cluster in Pseudomonas putida MT4 and evidence of oxidation of alkylphenols and alkylcatechols with mediumlength alkyl chain. J. Biosci. Bioeng. 102: 352–361.
Patel. V., S. Jain, and D. Madamwar (2012) Naphthalene degradation by bacterial consortium (DV-AL) developed from Alang-Sosiya ship breaking yard, Gujarat, India. Bioresour. Technol. 107: 122–130.
Duarte, M., A. Nielsen, A. Camarinha-Silva, R. Vilchez-Vargas, T. Bruls, M. L. Wos-Oxley, R. Jauregui, and D. H. Pieper (2017) Functional soil metagenomics: Elucidation of polycyclic aromatic hydrocarbon degradation potential following 12 years of in situ bioremediation. Env. Microbio. DOI: 10.1111/1462-2920.13756.
Martinkosky, L., J. Barkley, G. Sabadell, H. Gough, and S. Davidson (2017) Earthworms (Eisenia fetida) demonstrate potential for use in soil bioremediation by increasing the degradation rates of heavy crude oil hydrocarbons. Sci. Total Env. 580: 734–743.
Jeon, S., S. Hong, B. Kwon, J. Park, S. J. Song, J. P. Giesy, and J. S. Kim (2017) Assessment of potential biological activities and distributions of endocrine-disrupting chemicals in sediments of the west coast of South Korea. Chemosp. 168: 441–449.
Lobo, C. C., N. C Bertola, and E. M. Contreras (2013) Stoichiometry and kinetic of the aerobic oxidation of phenolic compounds by activated sludge. Bioresour. Technol. 136: 58–65.
Patel, V., S. Cheturvedula, and D. Madamwar (2012b) Phenanthrene degradation by Pseudoxanthomonas sp. DMVP2 isolated from hydrocarbon contaminated sediment of Amlakhadi canal, Gujarat, India. J. Haz. Mat. 201: 43–51.
Nuhoglu, A. and B. Yalcin (2005) Modelling of phenol removal in a batch reactor. Proc. Biochem. 40: 1233–1239.
Chung, T. P., H.Y. Tseng, and R. S. Juang (2003) Mass Transfer effect and intermediate detection for phenol degradation in immobilized Pseudomonas putida systems. Proc. Biochem. 38: 1497–1507.
Chang, Y. C., M. Venkateswar Reddy, H. Umemoto, Y. Sato, M. H. Kang, Y. Yajima, and S. Kikuchi (2015) Bio-Augmentation of Cupriavidus sp. CY-1 into 2,4-D contaminated soil: Microbial community analysis by culture dependent and independent techniques. PLoS ONE 10: 1–18.
Hong, S. H., J. S. Kim, J. G. Sim, and E. Y. Lee (2016) Isolation and characterization of the plant growth promoting Rhizobacterium, Arthrobacter scleromae SYE-3 on the Yam growth. KSBB J. 31: 58–65.
Shin, Y. J., C. H. Kang, and J. S. So (2016) Red pigment producing Serratia marcescens isolated from abalone. KSBB J. 31: 214–218.
Toledo, F. L., C. Calvo, B. Rodelas, and J. Gonzalez-Lopez (2006) Selection and identification of bacteria isolated from waste crude oil with polycyclic aromatic hydrocarbons removal capacities. Syst. Appl. Microbiol. 29: 244–252.
Kuang, Y., Y. Zhou, Z. Chen, M. Megharaj, and R. Naidu (2013) Impact of Fe and Ni/Fe nanoparticles on biodegradation of phenol by the strain Bacillus fusiformis (BFN) at various pH values. Bioresour. Technol. 136: 588–594.
Fayidh, M. A., S. Kallary, P. A. S. Babu, M. Sivarajan, and M. A. Sukumar (2015) A rapid and miniaturized method for the selection of microbial phenol degraders using colourimetric microtitration. Curr. Microbiol. 6: 898–906.
Devi, M. P., M. V. Reddy, A. Juwarkar, P. N. Sarma, and S. Venkata Mohan (2011) Effect of co-culture and nutrients supplementation on bioremediation of crude petroleum sludge. Clean–Soil, Air, Water 39: 900–907.
Ogata, Y., T. Toyama, N. Yu, X. Wang, K. Sei, and M. Ike (2013) Occurrence of 4-tert-butylphenol (4-t-BP) biodegradation in an aquatic sample caused by the presence of Spirodela polyrrhiza and isolation of a 4-t-BP-utilizing bacterium. Biodegradat. 24: 191–202.
Hahn, V., K. Sünwoldt, A. Mikolasch, and F. Schauer (2013) Biotransformation of 4-sec-butylphenol by Gram-positive bacteria of the genera Mycobacterium and Nocardia including modifications on the alkyl chain and the hydroxyl group. Appl. Microbiol. Biotechnol. 97: 8329–8339.
Wang, Z., Y. Yang, W. Sun, and S. Xie (2014) Biodegradation of nonylphenol by two alphaproteobacterial strains in liquid culture and sediment microcosm. Int. Biodet. Biodeg. 92: 1–5.
Tuan, N. N., Y. W. Lin, and S. L. Huang (2013) Catabolism of 4-alkylphenols by Acinetobacter sp. OP5: Genetic organization of the oph gene cluster and characterization of alkylcatechol 2, 3-dioxygenase. Bioresour. Technol. 131: 420–428.
Toyama, T., M. Murashita, K. Kobayashi, S. Kikuchi, K. Sei, Y. Tanaka, M. Ike, and K. Mori (2011) Acceleration of nonylphenol and 4-tert-Octylphenol degradation in sediment by Phragmites australis and associated Rhizosphere bacteria. Environ. Sci. Technol. 45: 6524–6530.
Chang, Y. C., S. Fuzisawa, M.V. Reddy, H. Kobayashi, E. Yoshida, Y. Yajima, T. Hoshino, and D.B. Choi (2016) Degradation of toxic compounds at low and medium temperature conditions using isolated Fungus. Clean–Soil, Air, Water 44: 992–1000.
Reddy, M. V., Y. Mawatari, Y. Yajima, C. Seki, T. Hoshino, and Y. C. Chang (2015) Poly-3-hydroxybutyrate (PHB) production from alkylphenols, mono and poly-aromatic hydrocarbons using Bacillus sp. CYR1: A new strategy for wealth from waste. Bioresour. Technol. 192: 711–717.
He, Z., C. Niu, and Z. Lu Z (2014) Individual or synchronous biodegradation of di-n-butyl phthalate and phenol by Rhodococcus ruber strain DP-2. J. Haz. Mat. 273: 104–109.
Maza-Márquez, P., M. V. Martínez-Toledo, V. González-López, B. Rodelas, B. Juárez-Jiménez, and M. Fenice (2013) Biodegradation of olive washing wastewater pollutants by highly efficient phenol-degrading strains selected from adapted bacterial community. Int. Biodet. Biodeg. 82: 192–198.
Bonfa, M. R. L., M. J. Grossman, F. Piubeli, E. Mellado, and L. R. Durrant (2013) Phenol degradation by halophilic bacteria isolated from hypersaline environments. Biodeg. 24: 699–709.
Venkateswar Reddy, M., Y. Mawatari, Y. Yajima, C. Seki, T. Hoshino, and Y. C. Chang (2015) Degradation and conversion of toxic compounds into useful bioplastics by Cupriavidus sp. CY-1: Relative expression of the PhaC gene under phenol and nitrogen stress. Green Chem. 17: 4560–4569.
Qi, J., B. Wang, J. Li, H. Ning, Y. Wang, W. Kong, and L. Shen (2008) Genetic determinants involved in the biodegradation of naphthalene and phenanthrene in Pseudomonas aeruginosa PAO1. Environ. Sci. Pollut. Res. 22: 6743–6749.
Masakorala, K., J. Yao, M. Cai, R. Chandankere, H. Yuan, and H. Chen (2013) Isolation and characterization of a novel phenanthrene (PHE) degrading strain Psuedomonas sp. USTB-RU from petroleum contaminated soil. J. Haz. Mat. 263: 493–500.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Reddy, M.V., Yajima, Y., Choi, D. et al. Biodegradation of toxic organic compounds using a newly isolated Bacillus sp. CYR2. Biotechnol Bioproc E 22, 339–346 (2017). https://doi.org/10.1007/s12257-017-0117-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-017-0117-0