Abstract
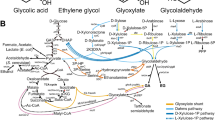
Ethylene is a major petrochemical for which biotechnological production methods are an attractive alternative. Here we use a system based on a bacterial ethylene forming enzyme (EFE) expressed in Saccharomyces cerevisiae. Metabolic modelling performed in a previous study identified re-oxidation of NADH as a factor limiting ethylene production in S. cerevisiae. In line with this, we here found that strains with multicopy plasmid expression of the heterologous oxidases nox and Aox1 led to significantly increased specific ethylene productivity, up 12 and 36%, respectively, compared to the control strain with empty plasmid. However the productivity and yield was only improved in the AOX expressing strain compared to that of the control strain. Both oxidase expressing strains also exhibited increased respiration rates compared to the reference strain, with specific oxygen consumption rates being roughly doubled in both strains. The AOX strain furthermore exhibited a significant increase in the EFE substrate 2-oxoglutarate formation compared to the reference strain, linking an improvement in ethylene production to both increased respiratory capacity and increased substrate availability, thereby corroborating our previous finding.
Similar content being viewed by others
References
Adams, D. O. and S. F. Yang (1979) Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA. 76: 170–174.
Yang, S. F. and N. E. Hoffman (1984) Ethylene biosynthesis and its regulation in higher-plants. Annu. Rev. Plant Phys. 35: 155–189.
Fukuda, H., T. Ogawa, and S. Tanase (1993) Ethylene production by micro-organisms. Adv. Microb. Physiol. 35: 275–306.
Nagahama, K., T. Ogawa, T. Fujii, and H. Fukuda (1992) Classification of ethylene-producing bacteria in terms of biosynthetic pathways to ethylene. J. Ferment. Bioeng. 73: 1–5.
Chen, X., Y. Liang, J. Hua, L. Tao, W. Qin, and S. Chen (2010) Overexpression of bacterial ethylene-forming enzyme gene in Trichoderma reesei enhanced the production of ethylene. Int. J. Biol. Sci. 6: 96–106.
Eckert, C., W. Xu, W. Xiong, S. Lynch, J. Ungerer, L. Tao, R. Gill, P. C. Maness, and J. Yu (2014) Ethylene-forming enzyme and bioethylene production. Biotechnol. Biofuels 7:33.
Ishihara, K., M. Matsuoka, Y. Inoue, S. Tanase, T. Ogawa, and H. Fukuda (1995) Overexpression and in vitro reconstitution of the ethylene-forming enzyme from Pseudomonas syringae. J. Ferment. Bioeng. 79: 205–211.
Ishihara, K., M. Matsuoka, T. Ogawa, and H. Fukuda (1996) Ethylene production using a broad-host-range plasmid in Pseudomonas syringae and Pseudomonas putida. J. Ferment. Bioeng. 82: 509–511.
Sakai, M., T. Ogawa, M. Matsuoka, and H. Fukuda (1997) Photosynthetic conversion of carbon dioxide to ethylene by the recombinant cyanobacterium, Synechococcus sp. PCC 7942, which harbors a gene for the ethylene-forming enzyme of Pseudomonas syringae. J. Ferment. Bioeng. 84: 434–443.
Tao, L., H. J. Dong, X. Chen, S. F. Chen, and T. H. Wang (2008) Expression of ethylene-forming enzyme (EFE) of Pseudomonas syringae pv. glycinea in Trichoderma viride. Appl. Microbiol. Biot. 80: 573–578.
Ungerer, J., L. Tao, M. Davis, M. Ghirardi, P. C. Maness, and J. P. Yu (2012) Sustained photosynthetic conversion of CO2 to ethylene in recombinant cyanobacterium Synechocystis 6803. Energ. Environ. Sci. 5: 8998–9006.
Pirkov, I., E. Albers, J. Norbeck, and C. Larsson (2008) Ethylene production by metabolic engineering of the yeast Saccharomyces cerevisiae. Metabol. Eng. 10: 276–280.
Johansson, N., K. O. Persson, P. Quehl, J. Norbeck, and C. Larsson (2014) Ethylene production in relation to nitrogen metabolism in Saccharomyces cerevisiae. FEMS Yeast Res. 14: 1110–1118.
Johansson, N., P. Quehl, J. Norbeck, and C. Larsson (2013) Identification of factors for improved ethylene production via the ethylene forming enzyme in chemostat cultures of Saccharomyces cerevisiae. Microbial. Cell Factor. 12:89.
Larsson, C., J. L. Snoep, J. Norbeck, and E. Albers (2011) Flux balance analysis for ethylene formation in genetically engineered Saccharomyces cerevisiae. IET Syst. Biol. 5: 245–251.
Vemuri, G. N., M. A. Eiteman, J. E. McEwen, L. Olsson, and J. Nielsen (2007) Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 104: 2402–2407.
Verduyn, C., E. Postma, W. A. Scheffers, and J. P. Van Dijken (1992) Effect of benzoic acid on metabolic fluxes in yeasts: A continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8: 501–517.
Dynesen, J., H. P. Smits, L. Olsson, and J. Nielsen (1998) Carbon catabolite repression of invertase during batch cultivations of Saccharomyces cerevisiae: the role of glucose, fructose, and mannose. Appl. Microbiol. Biotechnol. 50: 579–582.
Auzat, I., S. Chapuy-Regaud, G. Le Bras, D. Dos Santos, A. D. Ogunniyi, I. Le Thomas, J. R. Garel, J. C. Paton, and M. C. Trombe (1999) The NADH oxidase of Streptococcus pneumoniae: Its involvement in competence and virulence. Mol. Microbiol. 34: 1018–1028.
Akhter, S., H. C. McDade, J. M. Gorlach, G. Heinrich, G. M. Cox, and J. R. Perfect (2003) Role of alternative oxidase gene in pathogenesis of Cryptococcus neoformans. Infect. Immun. 71: 5794–5802.
Johnson, C. H., J. T. Prigge, A. D. Warren, and J. E. McEwen (2003) Characterization of an alternative oxidase activity of Histoplasma capsulatum. Yeast 20: 381–388.
Luttik, M. A., K. M. Overkamp, P. Kotter, S. de Vries, J. P. van Dijken, and J. T. Pronk (1998) The Saccharomyces cerevisiae NDE1 and NDE2 genes encode separate mitochondrial NADH dehydrogenases catalyzing the oxidation of cytosolic NADH. J. Biol. Chem. 273: 24529–24534.
Small, W. C. and L. McAlister-Henn (1998) Identification of a cytosolically directed NADH dehydrogenase in mitochondria of Saccharomyces cerevisiae. J. Bacteriol. 180: 4051–4055.
Larsson, C., I. L. Pahlman, R. Ansell, M. Rigoulet, L. Adler, and L. Gustafsson (1998) The importance of the glycerol 3-phosphate shuttle during aerobic growth of Saccharomyces cerevisiae. Yeast 14: 347–357.
Pahlman, I. L., L. Gustafsson, M. Rigoulet, and C. Larsson (2001) Cytosolic redox metabolism in aerobic chemostat cultures of Saccharomyces cerevisiae. Yeast 18: 611–620.
Rigoulet, M., H. Aguilaniu, N. Averet, O. Bunoust, N. Camougrand, X. Grandier-Vazeille, C. Larsson, I. L. Pahlman, S. Manon, and L. Gustafsson (2004) Organization and regulation of the cytosolic NADH metabolism in the yeast Saccharomyces cerevisiae. Mol. Cell Biochem. 256-257: 73–81.
Entian, K. D. and P. Kötter (2007) Yeast genetic strain and plasmid collections. pp. 629–666. In: I. Stansfield, and M. Stark (eds.). Methods in Microbiology. Elsevier.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johansson, N., Persson, K.O., Norbeck, J. et al. Expression of NADH-oxidases enhances ethylene productivity in Saccharomyces cerevisiae expressing the bacterial EFE. Biotechnol Bioproc E 22, 195–199 (2017). https://doi.org/10.1007/s12257-016-0602-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-016-0602-x