Abstract
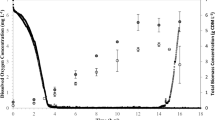
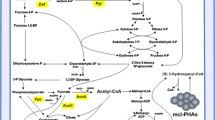
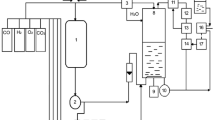
The production of medium chain length polyhydroxyalkanoates by Pseudomonas putida KT2440 from fatty acids leads to the loss of a large proportion of carbon. We studied the possibility of a shift of potentially available energy and carbon towards monitored residual growth during the production phase. A Fed-Batch culture achieving 125.6 g/L of total biomass containing 54.4% (g/g) of medium chain length polyhydroxyalkanoates was carried out leading to an overall experimental carbon yield of 0.7 Cmole/Cmole. The analysis of modeling fluxes deduced from experimental data indicated how carbon and reduced cofactors (NADH and FADH2) were managed to conclude that part of the carbon and reduced cofactors made available by polymer production were used in anabolic pathways. The strategy which consisted in coupled growth and medium chain length polyhydroxyalkanoate production enhanced the global yields compared to growth followed by a production phase. The understanding of carbon and energy fluxes distribution allowed deducing optimized culture strategy to perform the highest reported in the literature.
Similar content being viewed by others
References
Steinbuchel, A. and H. E. Valentin (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 128: 219–228.
Agnew, D. E. and B. F. Pfleger (2013) Synthetic biology strategies for synthesizing polyhydroxyalkanoates from unrelated carbon sources. Chem. Eng. Sci. 103: 58–67.
Lee, E. Y. and C. T. Choi (1997) Biosynthesis and biotechnological production of degradable polyhydroxyalkanoic acid. Biotechnol. Bioproc. Eng. 2: 1–10.
Sang Yup Lee (1996) High cell density cultivation of Pseudomonas oleovorans for the production of Poly(3-Hydroxyalkanoates). Biotechnol. Bioproc. Eng. 1: 51–53.
Abe, H., N. Ishii, S. Sato, and T. Tsuge (2012) Thermal properties and crystallization behaviors of medium-chain-length poly(3-hydroxyalkanoate)s. Polymer. 53: 3026–3034.
Koller, M., A. Salerno, M. Dias, and A. Reiterer (2010) Modern biotechnological polymer synthesis: A review. Food Technol. Biotechnol. 48: 255–269.
Jiang, G., D.J. Hill, M. Kovalczik, B. Johnston, G. Adamus, V. Irorere, and I. Radecka (2016) Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 17: 1157.
Liu, C. -C., L. -L. Zhang, B. Chen, and H. Yang (2016) Recent strategies for efficient production ofpolyhydroxyalkanaotes by micro-organisms. Lett. Appl. Microbiol. 62: 9–15.
Hazenberg, W. and B. Witholt (1997) Efficient production of medium-chain-length poly(3-hydroxyalkanoates) from octane by Pseudomonas oleovorans: Economic considerations. Appl. Microbiol. Biot. 48: 588–596.
Witholt, B. and B. Kessler (1999) Perspectives of medium chain length a versatile set of bacterial bioplastics. Curr. Opin. Biotech. 10: 279–285.
Steinbüchel, A. and T. Lütke-Eversloh (2003) Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 16: 81–96.
Kellerhals, M. B., B. Kessler, B. Witholt, A. Tchouboukov, and H. Brandl (2000) Renewable long-chain fatty acids for production of biodegradable medium-chain-length polyhydroxyalkanoates (mcl-PHAs) at laboratory and pilot plant scales. Macromol. 33: 4690–4698.
Grousseau, E., E. Blanchet, S. Deleris, M. G. E. Albuquerque, E. Paul, and J. L. Uribelarrea (2013) Impact of sustaining a controlled residual growth on polyhydroxybutyrate yield and production kinetics in Cupriavidus necator. Bioresour. Technol. 148: 30–38.
Brandl, H., R. A. Gross, R. W. Lenz, and R. C. Fuller (1988) Pseudomonas oleovorans as a source of poly(beta-Hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Microbiol. Biot. 54: 1977–1982.
Van Duuren, J. B. J. H., J. Puchalka, A. E. Mars, R. Bücker, G. Eggink, C. Wittmann, and V. A. P. M. dos Santos (2013) Reconciling in vivo and in silico key biological parameters of Pseudomonas putida KT2440 during growth on glucose under carbonlimited condition. BMC Biotechnol. 13: 93.
Blank, L. M., G. Ionidis, B. E. Ebert, B. Bühler, and A. Schmid (2008) Metabolic response of Pseudomonas putida during redox biocatalysis in the presence of a second octanol phase. FEBS J. 275: 5173–5190.
Chavarra, M., R. J. Kleijn, U. Sauer, K. Pflüger-Grau, and V. de Lorenzo (2012) Regulatory tasks of the Phosphoenolpyruvatephosphotransferase system of Pseudomonas putida in central carbon metabolism. mBio. 3: 2.
Escapa, I. F., J. L. Garcia, B. Bühler, L. M. Blank, and M. A. Prieto (2012) The polyhydroxyalkanoate metabolism controls carbon and energy spillage in Pseudomonas putida. Environ. Microbiol. 14: 1049–1063.
Latour, X. and P. Lemanceau (1998) Métabolisme carbon et énergétique des Pseudomonas spp fluorescents saprophytes oxydase positive. Agronomie. 17: 427–443.
Schulz, H. (1991) Beta oxidation of fatty acids. BBA -Lipid. Lipid. Met. 1081: 109–120.
Prieto, A., I. F. Escapa, V. Martinez, N. Dinjaski, C. Herencias, F. de la Pena, N. Tarazona, and O. Revelles (2016) A holistic view of polyhydroxyalkanoate metabolism in Pseudomonas putida. Environ. Microbiol. 18: 341–357.
Haywood, G. W., A. J. Anderson, and E. A. Dawes (1989) The importance of Phb-Synthase substrate-specificity in Polyhydroxyalkanoate synthesis by Alcaligenes eutrophus. FEMS Microbiol. Lett. 60: 245.
Sudesh, K., H. Abe, and Y. Doi (2000) Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 25: 1503–1555.
Weusthuis, R. A., G. N. M. Huijberts, and G. Eggink (1996) Production of MCL-Poly(3-hydroxyalknoates). International Symposium on Bacterial Polyhydroxyalkanoates. pp. 102–109. In: Eggink, G., A. Steinbüchel, Y. Poirier and B. Witholt (eds.). NRC Research Press, Ottawo, Canada.
Westerhoff, H. V., J. S. Lolkema, R. Otto, and K. J. Hellingwerf (1982) Thermodynamics of growth, Non-equilibrium thermodynamics of bacterial growth, The phenomenological and the mosaic approach. Biochim. Biophys. Acta 683: 181–220.
Khuwijitjaru, P., Y. Kimura, R. Matsuno, and S. Adachi (2004) Solubility of oleic and linoleic acids in subcritical water. Food Sci. Technol. Res. 10: 261–263.
Lee, S. Y., H. H. Wong, J. I. Choi, S. H. Lee, S. C. Lee, and C. S. Han (2000) Production of medium-chain-length polyhydroxyalkanoates by high-cell-density cultivation of Pseudomonas putida under phosphorus limitation. Biotechnol. Bioeng. 68: 466–470.
Aragao, G. (1996) Production de polyhydroxyalcanoates par Alcaligenes eutrophus: caractérisation cinétique et contribution l’optimisation de la mise en oeuvre des cultures. Ph. D. Thesis. Institut National des Sciences Appliquées, Toulouse, France.
Grousseau, E. (2012) Potentialités de production depolyhydroxyalcanoates chez Cupriavidus necator sur substrats de type acides gras volatils: études cinétiques et métaboliques. Ph.D. Thesis. Institut National des Sciences Appliquées, Toulouse, France.
De Eugenio, L. I., I. F. Escapa, V. Morales, N. Dinjaski, B. Galán, J. L. García, and M. A. Prieto (2010) The turnover of mediumchain-length polyhydroxyalkanoates in Pseudomonas putida KT2442 and the fundamental role of PhaZ depolymerase for the metabolic balance. Environ. Microbiol. 12: 207–221.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andin, N., Longieras, A., Veronese, T. et al. Improving carbon and energy distribution by coupling growth and medium chain length polyhydroxyalkanoate production from fatty acids by Pseudomonas putida KT2440. Biotechnol Bioproc E 22, 308–318 (2017). https://doi.org/10.1007/s12257-016-0449-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-016-0449-1