Abstract
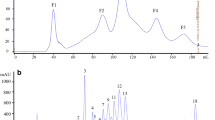
Cyclic lipopeptides were produced by Bacillus amyloliquefaciens subsp. plantarum strain BC32-1 that was isolated from yellow loess soil in the Jeonnam province of South Korea. Several lipopeptides were isolated from the bacteria using organic solvent extraction and reverse-phase high-performance liquid chromatography (RP-HPLC). Purified iturin-, surfactin-, and fengycin-type lipopeptides were identified using liquid chromatographymass spectrometry (LC-MS) analysis. Among the lipopeptides, C17-fengycin B showed strong antifungal activity against the phytopathogenic fungus, Fusarium oxysporum f. sp. radicis-lycopersici, and then the fengycin was further characterized by UV, Fourier transform-infrared spectroscopy (FT-IR), and LC-MS/MS analyses. C17-fengycin B was highly produced at quantities of up to 15 µg/mL at 37°C, whereas little amount of the fengycin was produced at 25°C. Purified C17-fengycin B inhibited mycelial growth of F. oxysporum with a minimal inhibitory concentration of 50 µg/mL. This study suggests that C17-fengycin B is a major antifungal component produced by the BC32-1 strain that could be used as an environmentally friendly agent to control the phytopathogenic F. oxysporum.
Similar content being viewed by others
References
Compant, S., B. Duffy, J. Nowak, C. Clement, and E. A. Barka (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 71: 4951–4959.
Ongena, M. and P. Jacques (2008) Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 16: 115–125.
Raaijmakers, J. M., I. de Bruijn, and M. J. de Kock (2006) Cyclic lipopeptide production by plant-associated Pseudomonas spp.: Diversity, activity, biosynthesis, and regulation. Mol. Plant Microbe Interact. 19: 699–710.
Katz, E. and A. L. Demain (1977) The peptide antibiotics of Bacillus: Chemistry, biogenesis, and possible functions. Bacteriol. Rev. 41: 449–474.
Schneider, T., A. Muller, H. Miess, and H. Gross (2014) Cyclic lipopeptides as antibacterial agents — potent antibiotic activity mediated by intriguing mode of actions. Int. J. Med. Microbial. 304: 37–43.
Mondol, M. A. M., H. J. Shin, and M. T. Islam (2013) Diversity of secondary metabolites from marine Bacillus Species: Chemistry and biological activity. Mar. Drugs. 11: 2846–2872.
Bernal, G., A. Illanes, and L. Ciampi (2002) Isolation and partial purification of a metabolite from a mutant strain of Bacillus sp with antibiotic activity against plant pathogenic agents. Electron. J. Biotechnol. 5: 12–20.
Delcambe, L., F. Peypoux, F. Besson, M. Guinand, and G. Michel (1977) Structure of iturin and iturin-like substances [proceedings]. Biochem. Soc. T. 5: 1122–1124.
Peypoux, F., J. M. Bonmatin, and J. Wallach (1999) Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 51: 553–563.
Raaijmakers, J. M., I. De Bruijn, O. Nybroe, and M. Ongena (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbial. Rev. 34: 1037–1062.
Vanittanakom, N., W. Loeffler, U. Koch, and G. Jung (1986) Fengycin-a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29–3. J. Antibiot. 39: 888–901.
Deleu, M., M. Paquot, and T. Nylander (2008) Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes. Biophys. J. 94: 2667–2679.
Romano, A., D. Vitullo, A. Di Pietro, G. Lima, and V. Lanzotti (2011) Antifungal lipopeptides from Bacillus amyloliquefaciens strain BO7. J. Nat. Prod. 74: 145–151.
Chen, X. H., A. Koumoutsi, R. Scholz, A. Eisenreich, K. Schneider, I. Heinemeyer, B. Morgenstern, B. Voss, W. R. Hess, O. Reva, H. Junge, B. Voigt, P. R. Jungblut, J. Vater, R. Süssmuth, H. Liesegang, A. Strittmatter, G. Gottschalk, and R. Borriss (2007) Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 25: 1007–1014.
Arguelles-Arias, A., M. Ongena, B. Halimi, Y. Lara, A. Brans, B. Joris, and P. Fickers (2009) Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb. Cell Fact. 8: 63.
Zhao, P., C. Quan, Y. Wang, J. Wang, and S. Fan (2014) Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. Spinaciae. J. Basic. Microbiol. 54: 448–456.
Nam, J., H. Yun, J. Kim, P. I. Kim, S. W. Kim, H. B. Lee, J. I. Kim, and C. W. Lee (2015) Isolation and NMR analysis of antifungal Fengycin A and B from Bacillus amyloliquefaciens subsp. plantarum BC32–1. Bull. Kor. Chem. Soc. 36: 1316–1321.
Benitez, L. B., R. V. Velho, M. P. Lisboa, L. F. Medina, and A. Brandelli (2010) Isolation and characterization of antifungal peptides produced by Bacillus amyloliquefaciens LBM5006. J. Microbiol. 48: 791–797.
Pecci, Y., F. Rivardo, M. G. Martinotti, and G. Allegrone (2010) LC/ESI-MS/MS characterisation of lipopeptide biosurfactants produced by the Bacillus licheniformis V9T14 strain. J. Mass Spectrom. 45: 772–778.
Szczechura, W., M. Staniaszek, and H. Habdas (2013) Fusarium oxysporum f. sp. radicis-lycopersici — the cause of fusarium crown and root rot in tomato cultivation. J. Plant. Prot. Res. 53: 172–176.
Kamilova, F., L. V. Kravchenko, A. I. Shaposhnikov, N. Makarova, and B. Lugtenberg (2006) Effects of the tomato pathogen Fusarium oxysporum f. sp. radicis-lycopersici and of the biocontrol bacterium Pseudomonas fluorescens WCS365 on the composition of organic acids and sugars in tomato root exudate. Mol. Plant Microbe Interact. 19: 1121–1126.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nam, J., Jung, M.Y., Kim, P.I. et al. Structural characterization and temperature-dependent production of C17-fengycin B derived from Bacillus amyloliquefaciens subsp. plantarum BC32-1. Biotechnol Bioproc E 20, 708–713 (2015). https://doi.org/10.1007/s12257-015-0350-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-015-0350-3