Abstract
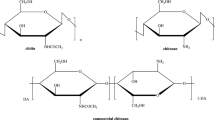
This study examined two gregarious Orthoptera species (Calliptamus barbarus and Oedaleus decorus) as potential sources of chitin. The chitin content of the dry weight of C. barbarus was 20.5 ± 0.7%, and it was 16.5 ± 0.7% for O. decorus. Furthermore, the yield of chitosan (70 ~ 75% deacetylation degree) from the grasshopper species was found to be 74 ~ 76%, which is close to the yield of commercial preparations obtained from the unused parts of crabs and shrimp. The chitin and chitosan obtained in this way were analyzed using FTIR, TGA, XRD and SEM techniques, and the antimicrobial properties of chitosans obtained from C. barbarus and O. decorus against pathogenic microorganisms of humans and fish were investigated using the disc diffusion and microdilution broth methods. The antimicrobial screening procedures indicated that the chitosan showed significant antimicrobial activity against all of the tested pathogenic microorganisms. The MBC or MFC values were determined to be 0.16 ~ 2.50 mg/mL. The IC50 values for the chitins obtained from C. barbarus and O. decorus were 10.68 ± 0.27 and 10.91 ± 0.96 mg/mL, respectively, which were greater than the value for butylated hydroxytoluene (BHT): 0.04 ± 0.01 mg/mL. These results suggest that these two species, which are currently considered to be pests because of over-breeding, are potentially alternative sources of chitin and chitosan, which are used in the food/feed industry for their antimicrobial and antioxidant properties.
Similar content being viewed by others
References
Brunner, E., H. Ehrlich, P. Schupp, R. Hedrich, S. Hunold, M. Kammer, S. Machill, S. Paasch, V.V. Bazhenov, D.V. Kurek, T. Arnold, S. Brockmann, M. Ruhnow, and R. Born (2009) Chitinbased scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 168: 539–547.
Ehrlich, H. (2010) Chitin and Collagen as Universal and Alter-native Templates in Biomineralization. Int. Geol. Rev. 52: 661–699.
Ehrlich, H., O.V. Kaluzhnaya, M.V. Tsurkan, A. Ereskowsky, K.R. Tabachnick, M. Ilan, A. Stelling, R. Galli, O.V. Petrova, S.V. Nekipelov, V.N. Sivkov, D. Vyalikh, R. Born, T. Behm, A. Ehrlich, L.I. Chernogor, S. Belikov, D. Janussen, V.V. Bazhenov, and G. Wörheide (2013a) First report on chitinous holdfast in sponges (Porifera). Proc. R. Soc. B. 280: 1471–2954.
Synowiecki, J. and N.A. Al-Khateeb (2003) Production, properties, and some new applications of chitin and its derivatives. Crit Rev Food Sci. 43: 145–171.
Rinaudo, M. (2006) Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 3: 603–632.
Park, B.K. and M.M. Kim (2010) Applications of chitin and its derivatives in biological medicine. Int. J. Mol. Sci. 11: 5152–5164.
Ehrlich H., P. Simon, M. Motylenko, M. Wysokowski, V.V. Bazhenov, R. Galli, A.L. Stelling, D. Stawski, M. Ilan, H. Stöcker, B. Abendroth, R. Born, T. Jesionowski, and D.C. Meyer (2013b) Extreme Biomimetics: Formation of Zirconium Dioxide Nanophase Using Chitinous Scaffolds under Hydrothermal Conditions. J. Mater. Chem. B 1: 5092–5099.
Yang J.K., I.L. Shih, Y.M. Tzeng, and S.L. Wang (2000) Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme Microb. Tech. 26: 406–413.
Nemtsev, S.V., O.Y. Zueva, M.R. Khismatullin, A.I. Albulov, and V.P. Varlamov (2004) Isolation of chitin and chitosan from honeybees. Appl. Biochem. Microbiol. 40: 39–43.
Zhang, M., A. Haga, H. Sekigushi, and S. Hirano (2000) Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 27: 99–105.
Majtan, J., K. Bilikova, O. Markovic, J. Grof, G. Kogan, and J. Simuth (2007) Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int. J. Biol. Macromol. 40: 237–241.
Ai, H., F. Wang, Q. Yang, F. Zhu, and C. Lei (2008) Preparation and biological activities of chitosan from the larvae of housefly, Musca domestica. Carbohyd. Polym. 72: 419–423.
Yen, M. and J. Mau (2006) Preparation of fungal chitin and chitosan from shiitakes tips. Fung. Sci. 21: 1–11.
Durkin, C.A., T. Mock, and E.V. Armbrust (2009) Chitin in diatomsand its association with the cell wall. Eukaryot Cell 8: 1038–1050.
Juárez-de la Rosa, B.A., P. Quintana, P.L. Ardisson, J.M. Yáñez-Limón, and J.J. Alvarado-Gil (2012) Effects of thermal treatments on the structure of two black coral species chitinous exoskeleton. J. Mater. Sci. 47: 990–998.
Bo, M., G. Bavestrello, D. Kurek, S. Paasch, E. Brunner, R. Born, R. Galli, A.L. Stelling, V.N. Sivkov, O.V. Petrova, D.V. Yalikh, K. Kummer, S.L. Molodtsov, D. Nowak, J. Nowak, and H. Ehrlich (2012) Isolation and identification of chitin in the black coral Parantipatheslarix (Anthozoa: Cnidaria). Int. J. Biol. Macromol. 51: 129–137.
Liu, S, J. Sun, L. Yu, C. Zhang, J. Bi, F. Zhu, M. Qu, C. Jiang, and Q. Yang (2012) Extraction and characterization of chitin from the beetle Holotrichia parallela motschulsky. Molecules 17: 4604–4611.
Ritchie, M.J. (1981) A taxonomic revision of the genus Oedaleus (Orthoptera: Acrididae). Bulletin of the British Museum (Natural History). Entomology Series 42: 83–183.
Bei-Bienko, G.J. and L.L. Mistshenko (1951) The grashopper of the fauna of the USSR and adjacent countries. Leningra, Moskova.
Blanchet, E., M. Lecoq, G.A. Sword, C. Pages, L. Blondin, C. Billot, R. Rivallan, A. Foucart, J.M. Vassal, A.M. Risterucci, and M.P. Chapuis (2012) Population structures of three Calliptamus spp. (Orthoptera:Acrididae) across the Western Mediterranean Basin. Eur. J. Entomol. 109: 445–445.
Kurita, K. (1998) Chemistry and application of chitin and chitosan. Polym. Degrad. Stabil. 59: 117–120.
Nam, K.S. (2001) Evaluation of the antimutagenic potential of chitosan oligosaccharide: Rec, Ames and Umu tests. Biotechnol. Lett. 23: 971–975.
Salmabi, K.A. and P.N. Seema (2013) Antibacterial potential of chitosan on pathogenic Gram positive cocci. Advanced BioTech. 12: 10–13.
Chung, Y.C., Y.P. Su, C.C. Chen, G. Jia, H.L. Wang, J.C.G. Wu, and J.G. Lin (2004) Relationship between antibacterial activity of chitosans and surface characteristics of cell wall. Acta Pharmacol. Sin. 25: 932–936.
No, H.K., N.Y. Park, S.H. Lee, and S.P. Meyers (2002) Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 74: 65–72.
Zhong, Z.M., R.G. Xing, S. Liu, L. Wang, S.B. Cai, and P.C. Li (2008) Synthesis of acyl thiourea derivatives of chitosan and their antimicrobial activities in vitro. Carbohyd. Res. 343: 566–570.
Jeon, Y.J, P.J. Park, and S.K. Kim (2001) Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohyd. Polym. 44: 71–76.
Kong, M., X.G. Chen, K. Xing, and H.J. Park (2010) Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 144: 51–63.
Lin, H.Y. and C.C. Chou (2004) Antioxidant activities of water-soluble disaccharide chitosan derivatives. Food Res. Int. 37: 883–889.
Yen, M.T., Y.H. Tseng, R.C. Li, and J.L. Mau (2007) Antioxidant properties of fungal chitosan from shiitake stipes. LWT-Food Sci. Technol. 40: 255–261.
Yen, M.T., J.H. Yang, and J.L. Mau (2008) Antioxidant properties of chitosan from crab shells. Carbohyd. Polym. 74: 840–844.
Charernsriwilaiwat, N., P. Opanasopit, T. Rojanarata, and T. Ngawhirunpat 2012. In vitro antioxidant activity of chitosan aqueous solution: Effect of salt form. LWT -Food Sci. Technol. 11(2): 235–242.
Baxter, A., M. Dillon, K.D.A. Taylor, and G.A.F. Roberts (1992) Improved method for i.r. determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 14: 166–169.
Murray, P.R., E.J. Baron, M.A. Pfaller, F.C. Tenover, and R.H. Yolke (1995) Manual of Clinical Microbiology. 6th ed., Washington, USA.
Chandrasekaran, M. and V. Venkatesalu (2004) Antibacterial and antifungal activity of Syzygium jambolanum seeds. J. Ethnopharmacol. 91: 105–108.
Kirby, A.J. and R.J. Schmidt (1997) The antioxidant activity of Chinese herbs for eczema and of placebo herbs. J. Ethnopharmacol. 56: 103–108.
Oyaizu, M. (1986) Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 44: 307–315.
Jiang, J.C. and Q. Xu (2006) Kinetics of heterogeneous deacetylation of β-chitin. Chem. Eng. Technol. 29: 511–516.
Minke, R. and J. Blackwell (1978) The structure of alpha-chitin. J. Mol. Biol. 120: 167–181.
Sajomsang W. and P. Gonil (2010) Preparation and characterization of α-chitin from cicadasloughs. Mater. Sci. Eng. C. 30: 357–363.
Singh, D.K. and A.R. Ray (1994) Graft copolymerization of 2-hydroxyethylmethacrylate onto chitosan films and their blood compatibility. J Appl Polym. Sci. 53: 1115–1121.
Qu, X., A. Wirsen, and A.C. Albertsson (2000) Effect of lactic/glycolic acid side chains on the thermal degradation kinetics of chitosan derivatives. Polymer 41: 4841–4847.
Peng, T. and M.F.A. Goosen (1994) Structural changes of pH-sensitive chitosan/polyether hydrogels in different pH solution. J. Polym. Sci. Pol. Chem. 32(3): 591–596.
Peniche, C., C. Elvira, and S.J. Roman (1998) Interpolymer complexes of chitosan and polymethacrylic derivatives of salicylic acid: preparation, characterization and modification by thermal treatment. Polymer 39: 6549–6554.
Paulino, A.T., J.I. Simionato, J.C. Garcia, and J. Nozaki (2006) Characterization of chitosan and chitin produced from silk worm chrysalides. Carbohydr. Polym. 64: 98–103.
Mohammed, M.H., P.A. Williams, and O. Tverezovskaya (2013) Extraction of chitin from prawn shells and conversion to low molecular mass Chitosan. Food Hydrocolloids 31: 166–171.
Kittur, F., H. Prashanth, K. Sankar, and R. Tharanathan (2002) Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Poly. 49: 185–193.
Jayakumar R., T. Egawa, T. Furuike, S.V. Nair, and H. Tamura (2009) Synthesis, characterization, and thermal properties of phosphorylated chitin for biomedical applications. Polym. Eng. Sci. 49: 844–849.
Yen, M.T., J.H. Yang, and J.L. Mau (2009) Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 75: 15–21.
Wang, Y., Y. Chang, L. Yu, C. Zhang, X. Xu, Y. Xue, Z. Li, and C. Xue (2013) Crystalline structure and thermal property characterization of chitin from Antarctickrill (Euphausia superba). Carbohydr. Polym. 92: 90–97.
Kucukgulmez, A., M. Celik, Y. Yanar, D. Sen, H. Polat, and A.E. Kadak (2011) Physicochemical characterization of chitosan extracted from Metapenaeus stebbingi shells. Food Chem. 126: 1144–1148.
No, H.K. and S.P.J. Meyers (1995) Preparation and characterization of chitin and chitosan-a review. Aquat. Food Prod. T. 4: 27–52.
Muzzarelli, R.A.A, M. Tomasetti, and P. Ilari (1994) Depolymerization of chitosan with the aid of papain. Enzyme Microb. Tech. 16: 110–114.
No, H.K., J.W. Nah, and S.P. Meyers (2003) Effect of time/temperature treatment parameters on depolymerization of chitosan. J. Appl. Polym. Sci. 87: 1890–1894.
No, H.K., S.H. Kim, S.H. Lee, N.Y. Park, and W. Prinyawiwatkul (2006) Stability and antibacterial activity of chitosan solutions affected by storage temperature and time. Carbohydr. Polym. 65: 174–178.
Raafat, D., K. von Bargen, A. Haas, and H.-G. Sahl (2008) Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 74(12): 3764–3773.
Islam, M.M.D, S.M.D. Masum, and K.R. Mahbub (2011) In vitro antibacterial activity of shrimp chitosan against Salmonella paratyphi and Staphylococcus aureus. Journal of Bangladesh Chemical Society 24(2): 185–190.
Tsai, G.J., W.H. Su, H.C. Chen, and C.L. Pan (2002) Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fisheries Sci. 68: 170–177.
Ing, L.Y., N.M. Zin, A. Sarwar, and H. Katas (2012) Antifungal activity of chitosan nanoparticles and correlation with their physical properties. International Journal of Biomaterials, Volume Article ID 632698, 9 pages. Doi:10.1155/2012/632698.
Douglas, L.J. (2003) Candida biofilms and their role in infection. Trends Microbiol. 11: 30–36.
Perea, S. and T.F. Patterson (1999) The role of antifungal susceptibility testing in the management of patients with invasive mycoses. Rev. Iberoam Micol. 16: 180–186.
Matsugo, S., M. Mizuie, M. Matsugo, R. Ohwa, H. Kitano, and T. Konishi (1998) Synthesis and antioxidant activity of water-soluble chitosan derivatives. Biochem. Mol. Biol. Int. 44(5): 939–948.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaya, M., Baran, T., Asan-Ozusaglam, M. et al. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol Bioproc E 20, 168–179 (2015). https://doi.org/10.1007/s12257-014-0391-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-014-0391-z