Summary
The annual meeting of the American Society of Medical Oncology (ASCO) was held as usual in Chicago, while the meeting focused on gastrointestinal tumors, ASCO-GI, was held in San Francisco. In particular, ASCO-GI included many phase III trials, the data of which have the potential to change the practice in the near future for tumors of the upper gastrointestinal tract (upper-GI), including tumors of the esophagus, gastroesophageal junction, stomach, and pancreas. Interestingly, ASCO and also the European Society for Medical Oncology (ESMO) offer the virtual plenary sessions under the motto “Today’s Science Can Wait.” As more physicians and scientists become comfortable with online tools in the post-pandemic era, presenting potential practice-changing data outside of annual meetings appears feasible. Here we present a summary of upper-GI tumor abstracts from two major ASCO meetings including virtual plenary series.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastroesophageal adenocarcinoma
KEYNOTE-859 study
KEYNOTE-859 is a double-blind, placebo-controlled phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for advanced human epidermal receptor 2 (HER2)-negative gastric and gastroesophageal junction adenocarcinoma (G/GEJC; [1]). Interim analyses were presented at the European Society for Medical Oncology (ESMO) virtual plenary series and further biomarker investigations were demonstrated at the American Society of Clinical Oncology (ASCO) annual meeting of 2023 [2, 3].
In total, 1579 patients were randomized to pembrolizumab + chemotherapy or placebo + chemotherapy. Baseline characteristics were balanced between arms. Median overall survival (OS) was 12.9 months with pembrolizumab + chemotherapy versus 11.5 months with placebo + chemotherapy (HR: 0.78, 95% CI: 0.70–0.87; p < 0.0001). Median progression-free survival (PFS) was 6.9 months versus 5.6 months (HR: 0.76, 95% CI: 0.67–0.85; p < 0.0001). The overall response rate (ORR) was 51.3% versus 42.0% (p = 0.00009).
Biomarker results were as following: 618 (78.2%) and 617 (78.2%) patients from pembrolizumab + chemotherapy and placebo + chemotherapy had a programmed death ligand-1 combined positive score (PD-L1 CPS) of ≥ 1. This was 35.3% and 34.3% for CPS ≥ 10, respectively. In the PD-L1 CPS ≥ 1 group, median OS was 13.0 months (95% CI: 11.6–14.2) for pembrolizumab + chemotherapy versus 11.4 months (95% CI: 10.5–12.0) for placebo + chemotherapy. In the PD-L1 CPS ≥ 10 population, median OS was 15.7 months (95% CI: 13.8–19.3) with pembrolizumab + chemotherapy versus 11.8 months (95% CI: 10.3–12.7) with placebo + chemotherapy. The median duration of response (DOR), PFS, and ORR were extended in CPS-positive arms when compared to the control group. Immune-related adverse events (irAE) were observed in 27.1% versus 9.3% of pembrolizumab + chemotherapy and placebo + chemotherapy arms, respectively.
Pembrolizumab in combination with predominantly oxaliplatin-based chemotherapy regimen seems to have a survival benefit in patients with gastroesophageal cancers in contrast to the Keynote-62 trial [4], where pembrolizumab was combined with a cisplatin-based chemotherapy. However, the magnitude of benefit of pembrolizumab seems to be modest in the overall patient population. Subgroup analyses suggested a greater benefit in CPS-positive subgroups, both in CPS ≥ 1 and ≥ 10 patients. Particularly for patients with higher CPS scores, the outcome measures including OS, PFS, ORR, and DOR were increased in a clinically relevant manner. The European Medicines Agency (EMA) approved pembrolizumab based on these results for patients with CPS ≥ 1 advanced or metastatic gastroesophageal tumors. This recommendation was implemented in the ESMO living guidelines for gastric cancer [5]. It is important to mention that the Checkmate-649 trial included a similar patient population with a different threshold for CPS [6]. Based on the results of the Checkmate-649 trial, immunotherapy with nivolumab together with chemotherapy became standard irrespective of CPS level in the United States. However, approval was restricted to CPS ≥ 5 by the EMA. Now, based on the current approval of pembrolizumab, the most debated patient group of CPS ≥ 1–4 seems to have access to immunotherapy in European countries.
SPOTLIGHT and GLOW studies
Human epidermal growth factor receptor 2 (HER2) has been the first and so far the only treatment target as first-line treatment of metastatic gastroesophageal adenocarcinoma [7]. CLDN18.2 proved to be a promising biomarker, which is expressed in normal and malignant gastric mucosa [8]. Previous phase I and phase II studies demonstrated encouraging anti-tumor activity of CLDN18.2 inhibitor zolbetuximab in patients with metastatic gastroesophageal cancer [9, 10]. Therefore, two additional phase III studies were conducted in order to elucidate the efficacy of zolbetuximab in larger cohorts.
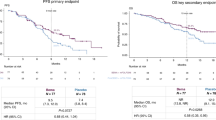
SPOTLIGHT trial investigated patients with CLDN18.2+ (moderate-to-strong membranous CLDN18 staining in ≥ 75% tumor cells by IHC)/HER2− unresectable or metastatic G/GEJC, and randomized patients to either zolbetuximab + mFOLFOX6 or placebo + mFOLFOX6 as first-line treatment [11, 12]. The primary endpoint was PFS. Secondary endpoints included OS, ORR, and safety. Among 2735 patients who were screened, 565 patients were randomized 1:1 to zolbetuximab + mFOLFOX6 (N = 283) or placebo + mFOLFOX6 (N = 282). It was found that PFS was statistically significantly improved with zolbetuximab + mFOLFOX6 (median 10.61 vs. 8.67 months, HR: 0.751, p = 0.0066). Furthermore, OS was also significantly improved (median 18.23 vs. 15.54 months, HR: 0.750, p = 0.0053). The ORR was similar between treatment arms. The most common treatment associated adverse events (TEAEs) with zolbetuximab + mFOLFOX6 were nausea (82.4% vs. 60.8% in zolbetuximab vs. placebo arms), vomiting (67.4% vs. 35.6%), and decreased appetite (47.0% vs. 33.5%); the incidence of serious TEAEs was similar between both arms (44.8% vs. 43.5%). Although the gastrointestinal side effects were remarkably higher in the experimental group, they usually occurred within the first 1–2 cycles and could mostly be successfully treated with appropriate medication.
In parallel to SPOTLIGHT, the GLOW study investigated a similar population; however, it utilized another chemotherapy backbone: CAPOX (combination of capecitabine and oxaliplatin; [13, 14]). Patients were randomized to zolbetuximab + CAPOX versus placebo + CAPOX. Both PFS (median 8.21 vs. 6.80 months, HR: 0.687, p = 0.0007) and OS (median 14.39 vs. 12.16 months, HR: 0.771, p = 0.0118) were significantly prolonged with zolbetuximab, and again ORR was not statistically significant. The toxicity profile was similar to that which was observed in SPOTLIGHT trial.
In conclusion, two international, randomized prospective phase III trials demonstrate that targeting CLDN18.2 with zolbetuximab combined with mFOLFOX6 or CAPOX significantly prolongs PFS and OS in first-line treatment of patients with CLDN18.2+/HER2− unresectable or metastatic G/GEJC. Thus, these treatment combinations have a strong potential to set a new standard-of-care option for this patient population. Based on the combined evidence of these two clinical trials, CLDN18.2 seems to be present in 38.4% of the entire population of advanced or metastatic gastroesophageal Her2-negative adenocarcinomas with varying geographic distribution [15]. A CPS of ≥ 5 was observed among 17.4% of the CLDN18.2-positive patients. The feasibility of the combination of anti-CLDN18.2 and anti-PD‑1 antibody is currently under investigation [16].
INTEGRATE IIa study
The previous phase II INTEGRATE trial investigated regorafenib as third-line treatment and showed improved outcome [17]. INTEGRATE IIa further investigated this product in a phase III setting and randomized patients with GC/GEJC to either regorafenib or placebo, who received at least two prior treatments for the metastatic disease [18]. The primary objective was OS in the whole study population. Median OS for regorafenib versus placebo was 4.5 versus 4.0 (HR: 0.70, 95% CI: 0.53–0.92, p = 0.011) in the whole study population, with a 12-month survival of 19% versus 6%. Median PFS was 1.8 months vs 1.6 months (HR: 0.52, 95% CI: 0.40–0.69, p = 0.0001). Regorafenib toxicity was similar to previously reported.
Regorafenib shows an activity as third-line and further-line treatment in a heavily treated patient population with gastroesophageal tumors. However, the magnitude of benefit driven from the treatment is low. It is important to mention that INTEGRATE IIb is an ongoing international randomized phase III trial for the same setting, where regorafenib will be combined with nivolumab and tested against a chemotherapy arm.
ATTRACTION-5 study
For gastroesophageal adenocarcinoma, immunotherapy is successfully implemented as first-line treatment worldwide, and third-line and further-line treatment in Asia [6, 19, 20]. This raised hopes that immunotherapy could also be useful for patients with resectable tumors. In Asian countries, adjuvant chemotherapy after gastrectomy and D2 lymphadenectomy is a widely used standard of care for stage III G/GEJ cancer [21]. However, novel treatment strategies are required, as recurrence rates are high. ATTRACTION‑5 was designed to answer this clinical situation and randomized patients with stage III G/GEJ cancer who had undergone D2 or more extended gastrectomy to either nivolumab + chemotherapy or placebo + chemotherapy [22]. This double-blind, randomized phase III study enrolled patients from Japan, Korea, Taiwan, and China. Adjuvant chemotherapy backbones included tegafur/gimeracil/oteracil (S-1) therapy or capecitabine plus oxaliplatin (CapeOX) therapy. The primary endpoint was centrally assessed relapse-free survival (RFS). A total of 755 patients could be randomized and the first data reported at the ASCO annual congress originated from a minimum follow-up of 36 months after the last patient was randomized. The primary endpoint RFS was not met (HR: 0.90; 95.72% CI: 0.69–1.18, p = 0.4363), with 3‑year RFS rates of 68.4% (95% CI: 63.0–73.2) in the nivolumab + chemotherapy arm and 65.3% (95% CI: 59.9–70.2) in the placebo + chemotherapy arm. The incidence of grade 3 ≥ TRAEs was 54.4% and 46.8% in the experimental and control arms, respectively.
Although immunotherapy has shown promise for patients with resectable gastroesophageal tumors, the ATTRACTION-5 trial was the first phase III trial to demonstrate negative results in this setting. This failure can have several causes: Immunotherapy was administered after removal of the tumor, which could result in insufficient memory T cell activation and ultimately no efficacy of immunotherapy. The treatment strategy of resectable stage III patients in Europe is significantly different from that of Asian patients because neoadjuvant treatment has been introduced as a safe and effective option for the former [23]. In contrast to ATTRACTION‑5, implementation of immunotherapy in the neoadjuvant setting is currently being investigated in numerous clinical trials [24, 25]. In addition, ATTRACTION‑5 did not perform a selection of patients based on the biomarker profile, as many clinical trials in the advanced setting showed better outcomes for patients with biomarker positivity. Furthermore, the chemotherapy backbone used in the ATTRACTION-5 trial is not recognized worldwide. In Europe and the United States, a triplet regimen is standard as perioperative treatment, and treatment with S1 is usually not feasible in these parts of the world.
Pancreatic adenocarcinoma
NAPOLI-3 study
Based on the phase III study NAPOLI‑1 investigating the role of NALIRI (liposomal irinotecan, 5‑fluorouracil [FU], and leucovorin) in the second-line setting after progression under a gemcitabine-based regimen [26], the phase III study NAPOLI‑3 presented at ASCO GI 2023 and recently published investigated the triplet chemotherapy regimen NALIRIFOX (liposomal irinotecan 50 mg/m2, 5‑FU 2400 mg/m2, leucovorin 400 mg/m2, and oxaliplatin 60 mg/m2) given on day 1 and 15 of a 28-day cycle compared to the combination of gemcitabine and nab-paclitaxel in the first-line setting in previously untreated pancreatic cancer patients [27]. After a median follow-up of 16.1 months, the primary endpoint of OS was 11.1 months in the triplet chemotherapy arm versus 9.2 months with gemcitabine and nab-paclitaxel (HR: 0.84; 95% CI: 0.71–0.99, p = 0.04). It was also shown that PFS was significantly longer with the triplet combination, with 7.4 versus 5.6 months (HR: 0.70, 95% CI: 0.59–0.84; p = 0.0001), and the ORR was quite comparable at 41.8% versus 36.2%. Comparing the toxicity profile of both regimens, no significant differences were observed with a comparable amount of grade 3/4 adverse events (AEs) comprising diarrhea, nausea, vomiting, and hypokalemia as the most frequently observed ones. Whether NALIRIFOX appears beneficial over the standard triplet regimen of FOLFIRINOX is difficult to answer since no phase III trials to date have compared these two regimens. However, when comparing the results of NALIRIFOX in the NAPOLI-3 trial with FOLFIRINOX of the PRODIGE-4/ACCORD-11 trial [28], median OS was completely similar with 11.1 months. With regard to the safety profile, 87% of patients experienced grade 3/4 AEs with NALIRIFOX according to the NAPOLI-3 trial, whereas about 76% had been described so far with FOLFIRINOX according to larger randomized studies. Grade 3/4 AEs seem to differ between the two regimens with diarrhea (20%), hypokalemia (15%), and neutropenia (14%) being the most frequently observed AE with NALIRIFOX and neutropenia (46%), fatigue (24%), and vomiting (15%) with FOLFIRINOX. Since there are no randomized data comparing FOLFIRINOX with gem/nab-paclitaxel, the data of NAPOLI‑3 support the NALIRIFOX regimen as the new standard of care in patients with a good performance status as first-line treatment.
References
Tabernero J, Bang YJ, Van Cutsem E, Fuchs CS, Janjigian YY, Bhagia P, et al. KEYNOTE-859: a Phase III study of pembrolizumab plus chemotherapy in gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021;17(22):2847–55.
Rha SY, Wyrwicz L, Weber PEY, Bai Y, Ryu MH, Lee J, et al. KEYNOTE-859 study of pembrolizumab plus chemotherapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Outcomes in the protocol-specified PD-L1-selected populations. J Clin Oncol. 2023;.
Rha SY, Wyrwicz LS, Weber PEY, Bai Y, Ryu MH, Lee J, et al. Pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Phase III KEYNOTE-859 study. Ann Oncol. 2023;.
Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6(10:1571–80.
Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–20.
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Rohde C, Yamaguchi R, Mukhina S, Sahin U, Itoh K, Tureci O. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49(9):870–6.
Sahin U, Tureci O, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32(5):609–19.
Tureci O, Sahin U, Schulze-Bergkamen H, Zvirbule Z, Lordick F, Koeberle D, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. 2019;30(9):1487–95.
Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401(10389):1655–68.
Shitara K, Lordick F, Bang YJ, Enzinger PC, Ilson DH, Shah MA, et al. Zolbetuximab + mFOLFOX6 as first-line (1L) treatment for patients (pts) with claudin-18.2+ (CLDN18.2+) / HER2− locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary results from phase 3 SPOTLIGHT study. J Clin Oncol. 2023;.
Shah MA, Shitara K, Ajani JA, Bang YJ, Enzinger P, Ilson D, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29(8):2133–41.
Xu RH, Shitara K, Ajani JA, Bang YJ, Enzinger PC, Ilson DH, et al. Zolbetuximab + CAPOX in 1L claudin-18.2+ (CLDN18.2+)/HER2− locally advanced (LA) or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary phase 3 results from GLOW. J Clin Oncol. 2023;.
Shitara K, Xu RH, Moran DM, Guerrero A, Li R, Pavese J, et al. Global prevalence of CLDN18.2 in patients with locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Biomarker analysis of two zolbetuximab phase 3 studies (SPOTLIGHT and GLOW). J Clin Oncol. 2023;.
Shitara K, Yamaguchi K, Shoji H, Matsangou M, Bhattacharya PP, Park JW, et al. Phase 2 trial of zolbetuximab in combination with mFOLFOX6 and nivolumab in patients with advanced or metastatic claudin 18.2-positive, HER2-negative gastric or gastroesophageal junction adenocarcinomas. J Clin Oncol. 2023;.
Pavlakis N, Sjoquist KM, Martin AJ, Tsobanis E, Yip S, Kang YK, et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol. 2016;34(23):2728–35.
Pavlakis N, Shitara K, Sjoquist KM, Martin AJ, Jaworski A, Yip S, et al. INTEGRATE IIa: A randomised, double-blind, phase III study of regorafenib versus placebo in refractory advanced gastro-oesophageal cancer (AGOC)—A study led by the Australasian Gastro-intestinal Trials Group (AGITG). J Clin Oncol. 2023;.
Chen LT, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2‑year update data. Gastric Cancer. 2020;23(3):510–9.
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–47.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Terashima M, Kang YK, Kim YW, Boku N, Chung HCC, Chen JS, et al. ATTRACTION-5: A phase 3 study of nivolumab plus chemotherapy as postoperative adjuvant treatment for pathological stage III (pStage III) gastric or gastroesophageal junction (G/GEJ) cancer. J Clin Oncol. 2023;.
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57.
Janjigian YY, Van Cutsem E, Muro K, Wainberg Z, Al-Batran SE, Hyung WJ, et al. MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol. 2022;18(20):2465–73.
Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, Tabernero J, Ilson DH, et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019;15(9):943–52.
Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545–57.
Wainberg ZA, Melisi D, Macarulla T, Pazo Cid R, Chandana SR, De La Fouchardiere C, et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. Lancet. 2023;402(10409):1272–81.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Ilhan-Mutlu declares following potential conflict of interests: Participation in advisory boards organized by MSD, Servier, Daiichi Sankyo, BMS and Astellas; lecture honoraria from Eli Lilly, Servier, BMS, MSD, Astellas and Daiichi Sankyo; consulting for Astellas, MSD, Amgen and Astra Zeneca; travel support from BMS, Roche, Eli Lilly and Daiichi Sankyo. E.S. Bergen has honoraria for lectures, consultation or advisory board participation from Servier.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Invited review article
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ilhan-Mutlu, A., Bergen, E.S. Upper-GI highlights from ASCO and ASCO-GI 2023 meetings: changing paradigm in treatment sequence. memo (2023). https://doi.org/10.1007/s12254-023-00944-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12254-023-00944-8