Summary
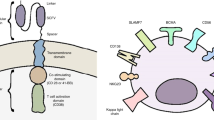
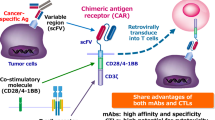
In this review we highlight the current developments in Chimeric antigen receptor (CAR) T cell therapy for myeloma. Two advanced products (idecabtagene vicleucel and ciltacabtagene autoleucel) targeting B‑cell maturation antigen have already been approved by the US Food and Drug Administration (FDA). In heavily pretreated myeloma patients, response rates between 82 and 98% and a median progression-free survival between 12 and > 24 months have been achieved. Currently these two products are being investigated in earlier lines of therapy. Beside BCMA, other targets have been selected for CAR T cell therapy in myeloma, with advanced constructs targeting two antigens. We highlight current strategies to improve CAR T cell effectiveness and safety and give a personal outlook where cellular immunotherapy in myeloma might be heading.
Similar content being viewed by others
References
Teoh PJ, Chng WJ. CAR T‑cell therapy in multiple myeloma: more room for improvement. Blood Cancer J. 2021;11(4):84.
Munshi NC, Anderson LD, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–16.
Martin T, Usmani SZ, Berdeja JG, et al. Updated results from CARTITUDE-1: phase 1b/2study of ciltacabtagene autoleucel, a B-cell maturation antigen—directed chimeric antigen receptor T cell therapy, in patients with relapsed/refractory multiple myeloma. Abstract. 63rd ASH Annual Meeting & Exposition. Vol. 549. 2021.
Mateos MV, Weisel K, Martin T, et al. Ciltacabtagene autoleucel for triple-class exposed multiple myeloma: adjusted comparisons of CARTITUDE-1 patient outcomes versus therapies from real-world clinical practice from the LocoMMotion prospective study. Abstract. 63rd ASH Annual Meeting & Exposition. Vol. 550. 2021.
Van de Donk NW, Themeli M, Usmani SZ. Determinants of response and mechanisms of resistance of CAR T‑cell therapy in multiple myeloma. Blood Cancer Discov. 2021;2(4):302–18.
Manier S, Ingegnere T, Escure G, et al. Current state and next-generation CAR‑T cells in multiple myeloma. Blood Rev. 2022; https://doi.org/10.1016/j.blre.2022.100929.
Atamaniuk J, Gleiss A, Porpaczy E, et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in patients with multiple myeloma. Eur J Clin Invest. 2012;42(9):953–60.
Mailankody S, Diamonte C, Fitzgerald L, et al. Phase I first-in-class trial of MCARH109, a G protein coupled receptor class C group 5 member D (GPRC5D) targeted CAR T cell therapy in patients with relapsed or refractory multiple myeloma. Abstract. 63rd ASH Annual Meeting & Exposition. Vol. 827. 2021.
Zah E, Nam E, Bhuvan V, et al. Systematically optimized BCMA/CS1 bispecific CAR‑T cells robustly control heterogeneous multiple myeloma. Nat Commun. 2020;11(1):2283.
Mei H, Li C, Jiang H, et al. A bispecific CAR‑T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol. 2021;14(1):161.
Van de Donk NW, Usmani SZ, Yong K. CAR T‑cell therapy for multiple myeloma: state of the art and prospects. Lancet Haematol. 2021;8(6):e446–e61. https://doi.org/10.1016/S2352-3026(21)00057-0.
Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T‑cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–24.
Mailankody S, Jakubowiak AJ, Htut M, et al. Orvacabtagene autoleucel (orva-cel), a B‑cell maturation antigen (BCMA)-directed CAR T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): update of the phase 1/2 EVOLVE study (NCT03430011). J Clin Oncol. 2020;38(15_suppl):8504.
Raje NS, Shah N, Jagannath S, et al. Updated clinical and correlative results from the phase I CRB-402 study of the BCMA-targeted CAR T cell therapy bb21217 in patients with relapsed and refractory multiple myeloma. Abstract. 63rd ASH Annual Meeting & Exposition. Vol. 548. 2021.
Mailankody S, Liedtke M, Sidana S, et al. Universal updated phase 1 data validates the feasibility of allogeneic anti-BCMA ALLO-715 therapy for relapsed/refractory multiple myeloma. Abstract. 63rd ASH Annual Meeting & Exposition. Vol. 651. 2021.
Pont MJ, Hill T, O’Cole G, et al. γ‑Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood. 2019;134(19):1585–97.
Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T‑cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–37.
Mestermann K, Giavridis T, Weber J, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med. 2019;11(499):eaau5907.
Van Oekelen O, Aleman A, Upadhyaya B, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR‑T cell therapy. Nat Med. 2021;27(12):2099–103.
Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–67.
Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215):215ra172.
Xie G, Dong H, Liang Y, et al. CAR-NK cells: a promising cellular immunotherapy for cancer. eBioMedicine. 2020;59:102975. https://doi.org/10.1016/j.ebiom.2020.102975.
Moscarelli J, Zahavi D, Maynard R, Weiner LM. The next generation of cellular immunotherapy: CAR-NK cells. Transplant Cell Ther. 2022; https://doi.org/10.1016/j.jtct.2022.06.025.
Carbone E, Neri P, Mesuraca M, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105(1):251–8.
Chu J, Deng Y, Benson DM, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28(4):917–27.
Jiang H, Zhang W, Shang P, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8(2):297–310.
Poels R, Drent E, Lameris R, et al. Preclinical evaluation of invariant natural killer T cells modified with CD38 or BCMA chimeric antigen receptors for multiple myeloma. Int J Mol Sci. 2021;22(3):1096.
Leivas A, Valeri A, Codoba L, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J. 2021;11(8):146.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N. Zojer received honoraria (ad boards, lectures) from Janssen, BMS, Celgene, Amgen, Sanofi, Takeda. M. Schreder received honoraria (ad boards, lectures) from Janssen, BMS, Amgen, Sanofi, AbbVie, Pfizer. H. Ludwig received honoraria (ad boards and/or lectures) from Janssen, Sanofi, Takeda, GSK, BMS, Abbvie.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zojer, N., Schreder, M. & Ludwig, H. CAR T cells in multiple myeloma: Where we stand and where we might be going. memo 15, 185–189 (2022). https://doi.org/10.1007/s12254-022-00825-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-022-00825-6