Abstract
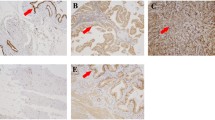
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors, with a high degree of malignancy and a poor prognosis. The aim of this study was to investigate the relationship between expression of pyruvate kinase M2 (PKM2) and prognosis in patients with HCC. The expression levels of PKM2 and PKM1 in 86 cases of HCC were detected by immunohistochemistry. An H score was used to evaluate the expression of PKM, and all patients were further divided into PKM high-expression and PKM low-expression groups. The relationship between PKM2 expression and the clinicopathological parameters and prognosis of patients were subsequently analyzed. Our data suggested that the expression level of PKM2 was significantly higher in HCC tissues than in adjacent tissues and the negatively expression of PKM1 in HCC tissues. Kaplan-Meier analysis revealed that PKM2 expression was strongly associated with survival in HCC patients (P = 0.001). The patients in the PKM2 high-expression group had significantly shorter survival times than the patients in the PKM2 low-expression group (hazard ratio for death, 2.358; 95% confidence interval [1.156, 4.812]; P = 0.018). In conclusion, these data indicate that PKM2 expression in HCC tissue samples can be used as a prognostic factor for patients with HCC and that high PKM2 expression is correlated with a poor prognosis in HCC patients.



Similar content being viewed by others
References
Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE (2012) Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology 55:476–482. https://doi.org/10.1002/hep.24710
Li H, Huang W, Luo R (2015) The microRNA-325 inhibits hepatocellular carcinoma progression by targeting high mobility group box 1. Diagn Pathol 10:117. https://doi.org/10.1186/s13000-015-0323-z
Zeng QA, Qiu J, Zou R, Li Y, Li S, Li B, Huang P, Hong J, Zheng Y, Lao X, Yuan Y (2012) Clinical features and outcome of multiple primary malignancies involving hepatocellular carcinoma: a long-term follow-up study. BMC Cancer 12:148. https://doi.org/10.1186/1471-2407-12-148
Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH, REVEAL-HBV Study Group (2006) Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama 295:65–73. https://doi.org/10.1001/jama.295.1.65
Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127:S5–S16
Ang SF, Ng ES, Li H, Ong YH, Choo SP, Ngeow J, Toh HC, Lim KH, Yap HY, Tan CK, Ooi LL, Cheow PC, Chung AY, Chow PK, Foo KF, Tan MH (2015) The Singapore liver cancer recurrence (SLICER) score for relapse prediction in patients with surgically resected hepatocellular carcinoma. PLoS ONE 10:e0118658. https://doi.org/10.1371/journal.pone.0128058
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362:1907–1917. https://doi.org/10.1016/S0140-6736(03)14964-1
Yu G, Yu W, Jin G, Xu D, Chen Y, Xia T, Yu A, Fang W, Zhang X, Li Z, Xie K (2015) PKM2 regulates neural invasion of and predicts poor prognosis for human hilar cholangiocarcinoma. Mol Cancer 14:193. https://doi.org/10.1186/s12943-015-0462-6
Yamada S, Fuchs BC, Fujii T, Shimoyama Y, Sugimoto H, Nomoto S, Takeda S, Tanabe KK, Kodera Y, Nakao A (2013) Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery 154:946–954. https://doi.org/10.1016/j.surg.2013.05.004
MacDonald MJ, Chang CM (1985) Pancreatic islets contain the M2 isoenzyme of pyruvate kinase. Its phosphorylation has no effect on enzyme activity. Mol Cell Biochem 68:115–120
Dombrauckas JD, Santarsiero BD, Mesecar AD (2005) Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry 44:9417–9429. https://doi.org/10.1021/bi0474923
Vaupel P, Harrison L (2004) Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 9:4–9. https://doi.org/10.1634/theoncologist.9-90005-4
Wong N, De Melo J, Tang D (2013) PKM2, a central point of regulation in cancer metabolism. Int J Cell Biol 2013:242513:1–11. https://doi.org/10.1155/2013/242513
Noguchi T, Inoue H, Tanaka T (1986) The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem 261:13807–13812
Eigenbrodt E, Reinacher M, Scheefers-Borchel U, Scheefers H, Friis R (1992) Double role for pyruvate kinase type M2 in the expansion of phosphometabolite pools found in tumor cells. Crit Rev Oncog 3:91–115
Mazurek S, Boschek CB, Hugo F, Eigenbrodt E (2005) Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol 15:300–308. https://doi.org/10.1016/j.semcancer.2005.04.009
Steinberg P, Klingelhoffer A, Schafer A, Wust G, Weisse G, Oesch F, Eigenbrodt E (1999) Expression of pyruvate kinase M2 in preneoplastic hepatic foci of N-nitrosomorpholine-treated rats. Virchows Arch 434:213–220. https://doi.org/10.1007/s004280050330
David CJ, Chen M, Assanah M, Canoll P, Manley JL (2010) HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 63:364–368. https://doi.org/10.1038/nature08697
Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L, Jiang F (2016) PKM2 and cancer: the function of PKM2 beyond glycolysis. Oncol Lett 11:1980–1986. https://doi.org/10.1038/nature08697
Li Z, Yang P, Li Z (2014) The multifaceted regulation and functions of PKM2 in tumor progression. Biochim Biophys Acta 1846:285–296. https://doi.org/10.1016/j.bbcan.2014.07.008
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452:230–233. https://doi.org/10.1038/nature06734
Tamada M, Suematsu M, Saya H (2012) Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res 18:5554–5561. https://doi.org/10.1158/1078-0432.CCR-12-0859
Schulze G (2000) The tumor marker tumor M2-PK: an application in the diagnosis of gastrointestinal cancer. Anticancer Res 20:4961–4964
Wong N, Yan J, Ojo D, De Melo J, Cutz JC, Tang D (2014) Changes in PKM2 associate with prostate cancer progression. Cancer Investig 32:330–338. https://doi.org/10.3109/07357907.2014.919306
Hardt PD, Ngoumou BK, Rupp J, Schnell-Kretschmer H, Kloer HU (2000) Tumor M2-pyruvate kinase: a promising tumor marker in the diagnosis of gastro-intestinal cancer. Anticancer Res 20:4965–4968
Fan F, Wu H, Liu Z, Hou X, Chen W, Wang A, Lu Y (2016) Nuclear PKM2 expression, an independent risk factor for ER after curative resection of hepatocellular carcinoma. Biomed Pharmacother 84:1858–1864. https://doi.org/10.1016/j.biopha.2016.10.108
Chen Z, Lu X, Wang Z, Jin G, Wang Q, Chen D, Chen T, Li J, Fan J, Cong W, Gao Q, He X (2015) Co-expression of PKM2 and TRIM35 predicts survival and recurrence in hepatocellular carcinoma. Oncotarget 6:2538–2548. https://doi.org/10.18632/oncotarget.2991
Xu Q, Liu X, Zheng X, Yao Y, Liu Q (2014) PKM2 regulates Gli1 expression in hepatocellular carcinoma. Oncol Lett 8:1973–1979. https://doi.org/10.3892/ol.2014.2441
Fan FT, Shen CS, Tao L, Tian C, Liu ZG, Zhu ZJ, Liu YP, Pei CS, Wu HY, Zhang L, Wang AY, Zheng SZ, Huang SL, Lu Y (2014) PKM2 regulates hepatocellular carcinoma cell epithelial-mesenchymal transition and migration upon EGFR activation. Asian Pac J Cancer Prev 15:1961–1970
Liu WR, Tian MX, Yang LX, Lin YL, Jin L, Ding ZB, Shen YH, Peng YF, Gao DM, Zhou J, Qiu SJ, Dai Z, He R, Fan J, Shi YH (2015) PKM2 promotes metastasis by recruiting myeloid-derived suppressor cells and indicates poor prognosis for hepatocellular carcinoma. Oncotarget 6:846–861. https://doi.org/10.18632/oncotarget.2749
Liu Y, Wu H, Mei Y, Ding X, Yang X, Li C, Deng M, Gong J (2017) Clinicopathological and prognostic significance of PKM2 protein expression in cirrhotic hepatocellular carcinoma and non-cirrhotic hepatocellular carcinoma. Sci Rep 7:15294. https://doi.org/10.1038/s41598-017-14813-y
Funding
This study was supported by the Gansu Province Natural Science Youth fund (No. 1506RJYA225).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Nothing to disclose.
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
Cairo University Ethical Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Human/Animal Rights Statement
This article contain all liver cancer tissues and corresponding normal adjacent samples were collected from 86 patients who had undergone routine surgery at the second hospital affiliated with Lanzhou University from January 2011 to December 2012.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, R., Li, L., Yang, J. et al. Overexpression of Pyruvate Kinase M2 in Tumor Tissues Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Pathol. Oncol. Res. 26, 853–860 (2020). https://doi.org/10.1007/s12253-019-00630-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00630-3