Abstract
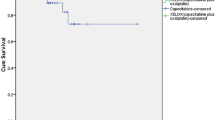
We decided to compare pathologic complete response (pCR) and disease-free survival (DFS) in rectal adenocarcinoma patients who received neoadjuvant chemoradiotherapy (CRT) with capecitabine plus oxaliplatin (XELOX) or capecitabine (Xeloda). In this study, patients with non-metastatic locally advanced rectal cancer (tumor stages of T2, T3, or T4) with or without lymph node involvement were retrospectively included. Patients received concomitant radiation (50.4–54 Gy external beam radiation in 28 to 30 fractions) and neoadjuvant therapy as either Xeloda (capecitabine, 2500 mg/m2 concomitantly with radiation therapy) (42patients) or XELOX [(oxaliplatin (50 mg/m2 intravenously once a week for five weeks) and capecitabine)] (72 patients). Surgery was done eight weeks after CRT. The endpoints were pCR (defined as no evidence of viable tumoral cells) and DFS (the interval from the initial treatment to the first tumor recurrence). Rectal sphincter preservation via low-anterior resection (LAR) was achieved in 73.8% of Xeloda group which was similar to XELOX group (70.8%), P = 0.61. pCR was documented in 11 (26.9%) of Xeloda group and 26 patients (36.1%) of XELOX group (P = 0.27). Tumor recurrence was recorded in 97 patients (85.1%). Mean (±SD) DFS was 52.13 (±31.92) months (median = 48 months). Mean (95% CI) DFS was 129.42 (110.19 to 148.64) in Xeloda group vs. 122.77 (110.72 to 134.83) in XELOX group (P = 0.74). Addition of oxaliplatin to capecitabine as neoadjuvant CRT for locally advanced rectal cancer did not result in improved pCR or better DFS.

Similar content being viewed by others
Data Availability
The raw data are available in statistical software.
Abbreviations
- APR:
-
abdomino-peritoneal resection
- CEA:
-
carcinoembryonic antigen
- CRT:
-
chemoradiotherapy
- DFS:
-
disease-free survival
- EUS:
-
endoultrasonography
- LAR:
-
low-anterior resection
- LN:
-
lymph node
- pCR:
-
pathologic complete response
References
Lorimer PD, Motz BM, Kirks RC, Boselli DM, Walsh KK, Prabhu RS, Hill JS, Salo JC (2017) Pathologic complete response rates after neoadjuvant treatment in rectal Cancer: An analysis of the National Cancer Database. Ann Surg Oncol 24(8):2095–2103
Mariette C, Brouquet A, Tzanis D, Laurenzi A, de la Rochefordiere A, Mariani P et al (2017) What is the impact of neoadjuvant chemoradiation on outcomes in gastro-intestinal cancer? J Visc Surg 154(3):185–195
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L et al (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355(11):1114–1123
Wasserberg N, Kundel Y, Purim O, Keidar A, Kashtan H, Sadot E, Fenig E, Brenner B (2014) Sphincter preservation in distal CT2N0 rectal cancer after preoperative chemoradiotherapy. Radiat Oncol 9:233
Kye BH, Kim HJ, Kim JG, Kim SH, Shim BY, Lee NS, Cho HM (2013) Short-term effects of neoadjuvant chemoradiation therapy on anorectal function in rectal cancer patients: a pilot study. Radiat Oncol 8:203
Schrag D (2013) Evolving role of neoadjuvant therapy in rectal cancer. Curr Treat Options Oncol 14(3):350–364
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17):1731–1740
Zhao L, Bai C, Shao Y, Guan M, Jia N, Xiao Y, Qiu H, Zhang F, Yang T, Zhong G, Chen S (2011) A phase II study of neoadjuvant chemoradiotherapy with oxaliplatin and capecitabine for rectal cancer. Cancer Lett 310(2):134–139
Hirsch BR, Zafar SY (2011) Capecitabine in the management of colorectal cancer. Cancer Manag Res 3:79–89
Haddad P, Miraie M, Farhan F, Fazeli MS, Alikhassi A, Maddah-Safaei A, Aghili M, Kalaghchi B, Babaei M (2017) Addition of oxaliplatin to neoadjuvant radiochemotherapy in MRI-defined T3, T4 or N+ rectal cancer: a randomized clinical trial. Asia Pac J Clin Oncol 13:416–422
Schmoll HJ, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E et al (2015) Capecitabine plus Oxaliplatin compared with fluorouracil/Folinic acid as adjuvant therapy for stage III Colon Cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 33(32):3733–3740
Hospers GA, Punt CJ, Tesselaar ME, Cats A, Havenga K, Leer JW et al (2007) Preoperative chemoradiotherapy with capecitabine and oxaliplatin in locally advanced rectal cancer. A phase I-II multicenter study of the Dutch colorectal Cancer group. Ann Surg Oncol 14(10):2773–2779
Gao YH, An X, Sun WJ et al (2014) Evaluation of capecitabine and oxaliplatin administered prior to and then concomitant to radiotherapy in high risk locally advanced rectal cancer. J Surg Oncol 109(5):478–482
Ricardi U, Racca P, Franco P, Munoz F, Fanchini L, Rondi N et al (2013) Prospective phase II trial of neoadjuvant chemo-radiotherapy with Oxaliplatin and Capecitabine in locally advanced rectal cancer (XELOXART). Med Oncol 30(2):581
Koeberle D, Burkhard R, von Moos R, Winterhalder R, Hess V, Heitzmann F, Ruhstaller T, Terraciano L, Neuweiler J, Bieri G, Rust C, Toepfer M (2008) Phase II study of capecitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advanced rectal cancer. Br J Cancer 98(7):1204–1209
Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu A, Melis M (2012) Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol 19(9):2822–2832
Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ et al (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11(9):835–844
Carlomagno C, Farella A, Bucci L, D'Armiento FP, Pesce G, Pepe S et al (2009) Neo-adjuvant treatment of rectal cancer with capecitabine and oxaliplatin in combination with radiotherapy: a phase II study. Ann Oncol 20(5):906–912
Rodel C, Liersch T, Hermann RM, Arnold D, Reese T, Hipp M et al (2007) Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol 25(1):110–117
O’Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S et al (2014) Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and bowel project trial R-04. J Clin Oncol 32(18):1927–1934
Gerard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL et al (2010) Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 28(10):1638–1644
Midgley RS, Yanagisawa Y, Kerr DJ (2009) Evolution of nonsurgical therapy for colorectal cancer. Nat Clin Pract Gastroenterol Hepatol 6(2):108–120
Sha A, Abadi S, Gill S (2017) Utilization of capecitabine plus oxaliplatin and 5-fluorouracil/folinic acid plus oxaliplatin in the adjuvant treatment of stage IIB and stage III colon cancer: a multi-Centre, retrospective, chart review study. J Oncol Pharm Pract 1078155217718381
Ostwal V, Engineer R, Ramaswamy A, Sahu A, Zanwar S, Arya S, Chopra S, Bal M, Patil P, Desouza A, Saklani A (2016) Surgical outcomes of post chemoradiotherapy unresectable locally advanced rectal cancers improve with interim chemotherapy, is FOLFIRINOX better than CAPOX? J Gastrointest Oncol 7(6):958–967
Liu F, Yang L, Wu Y et al (2016) CapOX as neoadjuvant chemotherapy for locally advanced operable colon cancer patients: a prospective single-arm phase II trial. Chin J Cancer Res 28(6):589–597
Yaghoubi A, Azadeh P, Sheibani K et al (2011) Comparison of 5FU-base Chemoradiation with and without Eloxatin on pathologic complete response in neoadjuvant Chemoradiation of rectal cancer. Iran J Pathol 6(3):110–116
Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Wolff RA, Eng C, Krishnan S, Janjan NA, Crane CH (2007) Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer 109(9):1750–1755
Wallin U, Rothenberger D, Lowry A, Luepker R, Mellgren A (2013) CEA - a predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis Colon Rectum 56(7):859–868
Zeng WG, Liang JW, Wang Z, Zhang XM, Hu JJ, Hou HR, Zhou HT, Zhou ZX (2015) Clinical parameters predicting pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Chin J Cancer 34(10):468–474
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations
Ethics approval and consent to participate: Ethical approval was obtained from our medical university research deputy. Informed written consent was obtained from patients.
Consent for Publication
The authors consent to publish the article.
Competing Interests
There is no competing interest with contents of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yaghobi Joybari, A., Azadeh, P., Babaei, S. et al. Comparison of Capecitabine (Xeloda) vs. Combination of Capecitabine and Oxaliplatin (XELOX) as Neoadjuvant CRT for Locally Advanced Rectal Cancer. Pathol. Oncol. Res. 25, 1599–1605 (2019). https://doi.org/10.1007/s12253-019-00587-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00587-3