Abstract
The present meta-analysis aimed to systematically evaluates the metastasis, clinical stage, and prognostic value regarding the expression levels of PVT1 in various cancers. Relevant literatures were searched in PubMed、Cochrane Library、Wed of science、Embase databases、Chinese National Knowledge Infrastructure and Wanfang from inception up to 22 August 2017. After data were extracted, a meta-analysis was performed using Review Manager 5.3 and Stata 12.0 software. The meta-analysis showed that high expression of PVT1 could predict more lymph node metastasis (LNM) (Odds ratio, OR = 2.83, 95% confidence interval, CI: 1.76–4.54, P < 0.0001), distant metastasis (DM) (OR = 3.60, 95% CI: 1.08–12.03, P = 0.04), advanced clinical stage (OR = 4.37, 95% CI: 3.45–5.54, P < 0.00001) and poor overall survival (Hazard ratio, HR = 2.08, 95% CI: 1.82–2.37, P < 0.00001)in cancer. Subgroup analysis in different systems also showed the same results, including respiratory system、digestive system、urinary system and other systems, especially in respiratory system (LNM, OR = 4.57, 95% CI: 2.41–8.68, P < 0.00001; clinical stage, OR = 5.59, 95% CI: 3.59–8.71, P < 0.00001; OS, HR = 2.43, 95% CI: 1.98–2.99, P < 0.00001). These results suggest that PVT1 could serve as a novel biomarker for metastasis, clinical stage and poor prognosis in various tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the incidence and mortality increased year by year, cancer is becoming a major public health problem and a leading cause of death worldwide. According to GLOBCAN 2012, there were 14.1 million new cancer cases and 8.2 million cancer deaths in 2012 worldwide [1]. In the United States, cancer is the second leading cause of death with an estimated 1,685,210 new cases and 595,690 deaths cancer in 2016 [2]. In China, cancer has been the leading cause of death with an estimated 4,292,000 new cases and 2,814,000 death cases in 2015 [3]. Lymph node metastasis, distant metastasis and clinical stage play an important role in the progression of cancer and are closely related to the prognosis of cancer. The presence of lymph node metastasis and distant metastasis also determines the treatment of cancer, such as surgery, radiotherapy or chemotherapy. Therefore, looking for molecular markers associated with metastasis, clinical stage and prognosis is becoming imminent for the therapy of cancer.
Long noncoding RNAs (lncRNAs) are a class of RNAs with a length greater than 200 nucleotides and no protein coding ability. Recently, with the rapid development of high throughput sequencing technology, lncRNAs have been found to be abnormally regulated in various types of cancer and played an indispensable role in the metastasis, advanced clinical stage and prognosis of cancer [4]. Plasmacytoma variant translocation1(PVT1) was first discovered in 2013 in human colorectal cancer and was a copy number amplification associated lncRNA which located on chromosome 8q24 and near MYC [5]. Accumulating evidence revealed that PVT1 was unregulated and played vital regulatory roles in a variety of cancers, including colorectal [6], pancreatic [7], breast and ovarian cancer [8]. High PVT1 expression was strongly correlated with clinicopathologic characteristics, such as metastasis, clinical stage and prognosis [9, 10]. However, since the results of the studies were not consistent (15 articles displayed positive results and 9 articles showed negative results) and small sample size in individual study, we collected relevant publications and performed a meta-analysis to investigate the relationship between PVT1 expression and metastasis, clinical stage or prognosis, aiming to further evaluate whether the PVT1 could be served as a potential molecular biomarker for cancers.
Material and Methods
Search Strategy and Literature Selection
We searched the electronic databases PubMed、Cochrane Library、Wed of science、Embase databases、Chinese National Knowledge Infrastructure and Wanfang, by using “PVT1 or plasmacytoma variant translocation1” as the keyword, in order to obtain potential articles referenced in the publications. Retrieval time for the last update is up to 22 August 2017.
Inclusion and Exclusion Criteria
Inclusion criteria for the articles were as the following: (1) Case-control studies that evaluate the relationship between PVT1 expression and metastasis, clinical stage or prognosis of patients in human cancer. (2) Patients were divided into high and low expression group according to PVT1 expression. (3) Related clinicopathologic parameters were described, such as lymph node metastasis、distant metastasis and clinical stage. (4) Related outcomes were reported, including overall survival (OS)、disease-free survival (DFS)、progression free survival (PFS)、recurrence free survival (RFS). (5) Sufficient data for calculating OR、HR and its corresponding 95% confidence intervals (CI).
Exclusion criteria for the articles were as follows: (1) Nonhuman research, reviews, editorials, expert opinions, letters and case reports. (2) Duplicate publications. (3) Studies without valuable data.
Date Extraction and Quality Assessment
Two investigators(YTH, CML)extracted and reviewed the essential data according to the inclusion and exclusion criteria independently. For each eligible study, we extracted the following information: first author, publication year, tumor type, country, total number of patients, detection method of PVT1 expression levels, number of high PVT1 expression group and low PVT1 expression group, number of patients with lymph node metastasis, distant metastasis and different clinical stage, follow-up duration, cut-off value, HRs as well as their 95% CIs. The quality of all eligible studies was assessed by two investigators (YCZ, ZYG) according to the Newcastle-Ottawa Scale independently. NOS scores ranged from 0 to 9 points, with higher scores indicated a better quality and all included eligible studies were assessed to be of high quality by using the NOS in this meta-analysis.
Statistical Analysis
The association between PVT1 and cancer metastasis, clinical stage, or prognosis was assessed by OR and HR with its corresponding 95% CI. The current meta-analysis was performed through Review Manager 5.3 and Stata 12.0 software. We use the Chi square-based Q test and I2 statistics evaluate the heterogeneity of the eligible studies. The random-effects model was used to analyze the results when heterogeneity was present (I2 > 50%, P < 0.05); while the fixed-effects model was applied for this meta-analysis when the heterogeneity was absent (I2 < 50%, P > 0.05). The potential publication bias was assessed with the Begg’s funnel-plot. P-value less than 0.05 were considered to be statistically significant.
Results
Literature Search and Study Characteristics
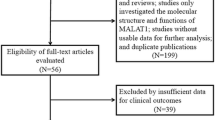
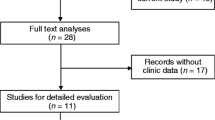
According to the inclusion and exclusion criteria, a total of 24 eligible studies [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] were screened upon an electrical search (Fig. 1). These studies included a total of 2212 patients; and the patient’s sample size ranges from 28 to 214 with the mean value of 92. Among the 24 studies, 6 studies focused on gastric cancer, 4 on non-small cell lung cancer and hepatocellular cancer respectively, one on lung cancer, colorectal cancer, pancreatic cancer, pancreatic ductal adenocarcinoma, renal cancer, bladder cancer, esophageal cancer, cervical cancer, epithelial ovarian cancer, and osteosarcoma respectively. All the diagnoses of lymph node metastasis, distant metastasis and tumor–node–metastasis (TNM) were based on pathology. In all of the included studies, the patients were divided into two groups: high and low expression of PVT1. All studies used qRT-PCR to detect the expression of PVT1. The main characteristics of the eligible studies were summarized in Tables 1 and 2.
Meta-Analysis Results
Association between PVT1 and LNM
15 studies reported 1197 patients with LNM based on different PVT1expression levels. The random-effects model was adopted as the significant heterogeneity (I2 = 65%, P = 0.0002). Analysis showed that the OR of high PVT1 expression group versus low PVT1 expression group was 2.83 (95% CI: 1.76–4.54, P < 0.0001) (Fig. 2), which revealed that a higher PVT1 expression predicted more LNM.
The subgroup analysis according to different systems in cancer types revealed a significant association between increased PVT1 expression and LNM in patients with respiratory system tumors (OR = 4.57, 95% CI: 2.41–8.68, P < 0.00001) and digestive system tumors (OR = 2.31, 95% CI: 1.18–4.49, P = 0.01). However, in urinary system tumors, the pooled result showed that cancer patients with high PVT1 expression were more likely to develop to LNM through no statistical significance was observed (OR = 1.09, 95% CI: 0.11–10.45, P = 0.94) (Fig. 2). This may be due to the too few literatures inclusion in the urinary system.
Association between PVT1 and DM
681 patients were included in 8 studies assessed the association between PVT1 expression and DM. The random-effects model was applied as the significant heterogeneity (I2 = 63%, P = 0.008). Analysis showed that high PVT1 expression was more prone to DM (OR = 3.60, 95% CI: 1.08–12.03, P = 0.04) (Fig. 3).
Association between PVT1 and Clinical Stage
20 studies contain 1485 patients with clinical stage were included. The fix-effect model was used as no heterogeneity existed (I2 = 0%, P = 0.81). Analysis showed the OR of 4.37 with 95% CI: 3.45–5.54 (P < 0.00001) (Fig. 4), which revealed that a higher PVT1 expression was predictive of advanced clinical stage.
The subgroup analysis according to different systems in cancer types revealed a significant association between increased PVT1 expression and advanced clinical stage in patients with respiratory system tumors (OR = 5.59, 95% CI: 3.59–8.71, P < 0.00001), digestive system tumors (OR = 3.59, 95% CI: 2.66–4.83, P < 0.00001) and other system tumors (OR = 8.75, 95% CI: 3.61–21.20, P < 0.00001) (Fig. 4).
Association between PVT1 and OS
16 studies reporting 1640 patients with OS were included according to different PVT1 expression levels. The fixed-effect model was used as the small heterogeneity existed (I2 = 38%, P = 0.06). Data of pooled HRs (HR = 2.08, 95% CI: 1.82–2.37, P < 0.00001) (Fig. 5) showed high PVT1 expression correlated with a worse survival.
The subgroup analysis also revealed a significant association between increased PVT1 expression and OS in patients with respiratory system tumors (HR = 2.43, 95% CI: 1.98–2.99, P < 0.00001), digestive system tumors (HR = 1.80, 95% CI: 1.43–2.26, P < 0.00001) and other system tumors (HR = 1.94, 95% CI: 1.47–2.55, P < 0.00001) (Fig. 5).
In additional, we investigated the association between PVT1 expression and DFS, PFS or RFS, respectively. The results revealed that significant negative association between PVT1 expression levels and DFS (HR = 1.87, 95% CI: 1.40–2.49, P < 0.0001), PFS (HR = 1.85, 95% CI: 1.47–2.32, P < 0.00001) or RFS (HR = 1.76, 95% CI: 1.19–2.61, P = 0.005) were existed. All the results were listed in the Table 3.
Publication Bias and Sensitivity Analysis
We use Egger’s test and funnel plot evaluate the publication bias of the present meta-analysis. Egger’s test (P = 0.728) revealed that there was no publication bias in analysis of LNM, and funnel plot (Fig. 6) showed no evidence of obvious asymmetry for LNM. Additionally,Similar results were also shown in the clinical stage and prognosis groups. Sensitivity analysis was carried out to evaluate the influence of a single study on the overall meta-analysis results by removing one study at a time in total population. When each study was omitted sequentially, the results were not significantly altered in this meta-analysis (Fig 7).
Discussion
PVT1 was a novel lncRNA which was first discovered in 2013 in human colorectal cancer. In recent years, a growing number of studies have shown that PVT1 upregulated in several cancers. The further comprehensive mechanism between PVT1 and cancers was reported in continuance. PVT1 involved in tumor proliferation, invasion, migration and apoptosis and played an important role in tumor progression, metastasis and prognosis. In order to combine previous research results about PVT1 and cancers to arrive at a summary conclusion, we clarified the relationships between PVT1 expression levels and metastasis, clinical stage or prognosis in cancers in this meta-analysis. As far as we know, this is the first meta-analysis providing comprehensive insights into the correlation of PVT1 and cancer clinical stage. Results showed that the risk of lymph node metastasis and distant metastasis in high PVT1 expression group was 2.83 and 3.60 folds than those with low PVT1 expression group, respectively. The risk of developing into advanced clinical stage in PVT1 over expression patients was 4.37 times higher than those with low PVT1expression. PVT1 over expression patients with poor prognosis were 2.08 times lower in patients with low PVT1 expression. The same results were also shown in the subgroup analysis of different system tumors.
First, in respiratory system tumors, Wan et al. found that Over expression PVT1 inhibited the expression of LATS2 by binding to enhancer of zeste homolog 2 (EZH2) and promoted cell proliferation in non-small cell lung cancer [15]. Digestive system tumors such as in esophageal cancer, PVT1 upregulation decreased E-cadherinexpression and increased N-cadherin and vimentin expression, and induced epithelial-to-mesenchymal transition (EMT) process [12]. In gastric cancer, Xu et al. suggested that PVT1 facilitated gastric cancer cell proliferation and metastasis, and fulfilled its oncogenic functions in a FOXM1-mediated manner [26]. In hepatocellular carcinoma, Lan et al. demonstrated that PVT1 over expression was significantly correlated with vascular invasion, liver cirrhosis and TNM stage. Mechanism studies showed that PVT1 served as an endogenous sponge for miR-186-5p to reduce its inhibiting effect on yes-associated protein 1 and thus promoted the tumorigenesis of hepatocellular carcinoma [29]. In colorectal cancer, Takahashi et al. demonstrated high PVT1 expression exhibited greater lymph node metastasis, venous invasion and a poor OS compared with low PVT1 expression [21]. In pancreatic carcinoma, Zhao et al. found that PVT1 functions as an endogenous ‘sponge’ by competing for miR-448 binding to regulate the miRNA target SERBP1and therefore promotes the proliferation and migration of pancreatic carcinoma cells [28]. Furthermore, PVT1 expression levels were significantly correlated with metastasis and advanced clinical stage in urinary system tumors, such as bladder cancer and renal carcinoma. Huang et al. showed that high PTV1 expression was correlated with lymph node metastasis, advanced TNM stage and shorter OS. Knock down PVT1 decreased cell proliferation and enhanced cell apoptosis. Finally, PVT1 also functioned as critical regulator in the prognosis of other system tumors, including cervical carcinoma, ovarian cancer and osteosarcoma. Song et al. suggested that high PVT1 expression predicted poor prognosis. PVT1 over expression increased glucose uptake, lactate production, and the expression of HK2 in osteosarcoma cells. PVT1 could act as molecular sponge to repress the expression of miR-497 and promote the development of osteosarcoma [30].
To summarize, although mechanisms of PVT1 acted were not the same in various cancers, many similarities were still existed. First, PVT1 played a key role in cancers by interacting with miRNAs. An inverse association was observed between PVT1 and miR-186 expression levels which was observed in gastric cancer and hepatocellular cancer, while PVT1 functions as an endogenous ‘sponge’ by competing for miR-448 in pancreatic cancer. Second, PVT1 could also alter the expression of certain genes, including bind to EZH2 in non-small cell lung cancer and gastric cancer. PVT1 could interact with FOXM1 directly and increase its protein expression in gastric cancer and increased glucose uptake, lactate production, and the expression of HK2 in osteosarcoma cancer. Finally, PVT1 also affected cell cycle progression, such as promoted cell proliferation, migration and invasion and inhibited cell apoptosis in various cancers. In general, PVT1 expression was significantly associated with metastasis, clinical stage, and poor prognosis in various types of cancer in different systems.
Otherwise, as with other meta-analysis, it should be acknowledged that some limitations existed in this meta-analysis. First, the cutoff values of PVT1 high expression and low expression were lack of uniform standard in different types of cancer, which may result in some heterogeneity and affect the results of the study. Second, different studies have different postoperative regimens that may have a significant impact on OS, DFS, PFS and RFS. Third, since most studies report positive results and negative results are rarely published, the results of this study may overestimate the effect of PVT1 on cancers to a certain extent. Although there are some limitations, but this meta-analysis still has its noteworthy advantages. First, 24 literatures including a total of 2212 cases were included in this meta-analysis. The sample size included was the largest, which significantly improved the statistical efficiency and accuracy of the test. Second, the search databases were the most and cancer types were the most comprehensive in this meta-analysis compared with the previous reports. Third, lymph node metastasis and distant metastasis, OS、DFS、PFS and RFS were included in this study, which made the results more complete and comprehensive. Finally, the inclusion and exclusion criteria were more stringent and the quality of the literatures incorporated was higher.
In conclusion, despite the limitations described above, our meta-analysis reveals that upregulated PVT1 is significantly correlated with more metastasis, advanced clinical stage and poor prognosis in patients with various cancers. Furthermore, the significance of PVT1 in the metastasis, clinical stage and prognosis of respiratory system tumors is more obvious and can be used as a potential molecular marker to evaluate the prognosis of cancer. Nevertheless, the indication in the urinary system is relatively weak as the fewer samples; suggesting that we need to incorporate more studies in the urinary system tumors to validate this result.
Abbreviations
- lncRNA :
-
long noncoding RNA
- PVT1 :
-
plasmacytoma variant translocation1
- LNM :
-
lymph node metastasis
- DM :
-
distant metastasis
- OR :
-
odds ratio
- CI :
-
confidence interval
- HR :
-
hazard ratio
- OS:
-
overall survival
- DFS :
-
disease-free survival
- PFS :
-
progression free survival
- RFS :
-
recurrence free survival
- TNM :
-
tumor–node–metastasis
- CNKI :
-
Chinese National Knowledge Infrastructure
- NSCLC :
-
non-small cell lung cancer
- SCLC :
-
small cell lung cancer
- GC :
-
gastric cancer
- PDAC :
-
pancreatic ductal adenocarcinoma
- CRC :
-
colorectal cancer
- HCC :
-
hepatocellular carcinoma
- EC :
-
esophageal cancer
- RC :
-
renal carcinoma
- BC :
-
bladder cancer
- PC :
-
pancreatic carcinoma
- EOC :
-
epithelial ovarian cancer
- OC :
-
osteosarcoma
- qRT-PCR :
-
quantitative real-time PCR
- NA :
-
not available
- EZH2 :
-
zeste homolog 2
- EMT :
-
epithelial-to-mesenchymal transition
References
GLOBOCAN (2012) Estimated Cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/burden_sel.aspx.[J]
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Chen W, Zheng R, Baade PD et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132
Yang B, Luo T, Zhang M et al (2017) The novel long noncoding RNA RP11-357H14.17 acts as an oncogene by promoting cell proliferation and invasion in diffuse-type gastric cancer. Onco Targets Ther 10:2635–2643
Cui M, You L, Ren X et al (2016) Long non-coding RNA PVT1 and cancer. Biochem Biophys Res Commun 471:10–14
Guo K, Yao J, Yu Q et al (2017) The expression pattern of long non-coding RNA PVT1 in tumor tissues and in extracellular vesicles of colorectal cancer correlates with cancer progression. Tumour Biol 39:1010428317699122
Wu BQ, Jiang Y, Zhu F et al (2017) Long noncoding RNA PVT1 promotes EMT and cell proliferation and migration through downregulating p21 in pancreatic Cancer cells. Technol Cancer Res Treat 1:1533034617700559
Guan Y, Kuo WL, Stilwell JL et al (2007) Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res 13:5745–5755
Huang T, Liu HW, Chen JQ et al (2017) The long noncoding RNA PVT1 functions as a competing endogenous RNA by sponging miR-186 in gastric cancer. Biomed Pharmacother 88:302–308
Huang C, Liu S, Wang H et al (2016) LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res 8:5025–5034
Zhuang C, Li J, Liu Y et al (2015) Tetracycline-inducible shRNA targeting long non-coding RNA PVT1 inhibits cell growth and induces apoptosis in bladder cancer cells. Oncotarget 6:41194–41203
Zheng X, Hu H, Li S (2016) High expression of lncRNA PVT1 promotes invasion by inducing epithelial-to-mesenchymal transition in esophageal cancer. Oncol Lett 12:2357–2362
Iden M, Fye S, Li K et al (2016) The lncRNA PVT1 contributes to the cervical Cancer phenotype and associates with poor patient prognosis. PLoS One 11
Cui D, Yu CH, Liu M et al (2016) Long non-coding RNA PVT1 as a novel biomarker for diagnosis and prognosis of non-small cell lung cancer. Tumour Biol 37:4127–4134
Wan L, Sun M, Liu GJ et al (2016) Long noncoding RNA PVT1 promotes non-small cell lung Cancer cell proliferation through epigenetically regulating LATS2 expression. Mol Cancer Ther 15:1082–1094
Yang YR, Zang SZ, Zhong CL et al (2014) Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol 7:6929–6935
Ding J, Li D, Gong M et al (2014) Expression and clinical significance of the long non-coding RNA PVT1 in human gastric cancer. Onco Targets Ther 7:1625–1630
Huang C, Yu W, Wang Q et al (2015) Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med 106:143–149
Kong R, Zhang EB, Yin DD et al (2015) Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol Cancer 14:015–0355
Ren XX, Xiao YB, Zhang L et al (2016) Expression and clinical significance of PVT1 gene in blood of the patients with gastric cancer. Chin J Cancer Biother 23:4
Takahashi Y, Sawada G, Kurashige J et al (2014) Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer 110:164–171
Wang F, Yuan JH, Wang SB et al (2014) Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology 60:1278–1290
Yuan CL, Li H, Zhu L et al (2016) Aberrant expression of long noncoding RNA PVT1 and its diagnostic and prognostic significance in patients with gastric cancer. Neoplasma 63:442–449
Huang Y, Mu ZY, Lin HL et al (2015) Expression and clinical significance of lncRNA PTV1 in renal carcinoma tissues. Shandong Med 55:3
Ding C, Yang Z, Lv Z et al (2015) Long non-coding RNA PVT1 is associated with tumor progression and predicts recurrence in hepatocellular carcinoma patients. Oncol Lett 9:955–963
Xu MD, Wang Y, Weng W et al (2017) A positive feedback loop of lncRNA-PVT1 and FOXM1 facilitates gastric Cancer growth and invasion. Clin Cancer Res 23:2071–2080
Martini P, Paracchini L, Caratti G et al (2017) lncRNAs as novel indicators of Patients' prognosis in stage I epithelial ovarian Cancer: a retrospective and multicentric study. Clin Cancer Res 23:2356–2366
Zhao L, Kong H, Sun H et al (2017) LncRNA-PVT1 promotes pancreatic cancer cells proliferation and migration through acting as a molecular sponge to regulate miR-448. J Cell Physiol 28:26072
Lan T, Yan X, Li Z et al (2017) Long non-coding RNA PVT1 serves as a competing endogenous RNA for miR-186-5p to promote the tumorigenesis and metastasis of hepatocellular carcinoma. Tumour Biol 39:1010428317705338
Song J, Wu X, Liu F et al (2017) Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochem Biophys Res Commun 490:217–224
Wu D, Li Y, Zhang H et al (2017) Knockdown of Lncrna PVT1 enhances Radiosensitivity in non-small cell lung Cancer by sponging Mir-195. Cell Physiol Biochem 42:2453–2466
Gou X, Zhao X, Wang Z (2017) Long noncoding RNA PVT1 promotes hepatocellular carcinoma progression through regulating miR-214. Cancer Biomark 31 CBM-170331
Acknowledgements
We would like to thank the authors of the primary studies and the Fourth Hospital of Hebei Medical University (Shijiazhuang, China) for their generous help. The work was fully supported by all of them.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, C., Jin, J., Liang, D. et al. Long Noncoding RNA PVT1 as a Novel Predictor of Metastasis, Clinicopathological Characteristics and Prognosis in Human Cancers: a Meta-Analysis. Pathol. Oncol. Res. 25, 837–847 (2019). https://doi.org/10.1007/s12253-018-0451-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-0451-3