Abstract
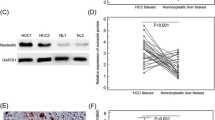
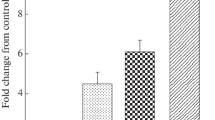
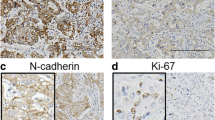
Hepatocellular carcinoma (HCC) is the most common type of primary malignant tumor in the liver. One of the main features of cancer survival is the generalized loss of growth control exhibited by cancer cells, and Miki is a protein related to the immunoglobulin superfamily that plays an important role in mitosis. We aim to study protein expression levels of Miki in non-tumoral liver and 20 HCCs recruited from a Pathology Department. Clinical information was also obtained. A tissue microarray was performed, and immunohistochemical techniques applied to study protein expression levels of Miki. In normal liver, Miki was weakly expressed, showing nuclear staining in the hepatocytes. Cirrhotic areas and HCCs showed a variety of staining patterns. Most HCC samples showed positive expression, with three different staining patterns being discernible: nuclear, cytoplasmic and mixed. Statistical analysis showed a significant association between grade of differentiation, Ki-67 proliferative index, survival rates and staining patterns. This study has revealed the positive expression of Miki in normal liver, cirrhotic areas and HCCs. Three different staining patterns of Miki expression with clinical relevance were noted in HCCs.



Similar content being viewed by others
References
Stoot JH, Coelen RJ, De Jong MC, Dejong CH (2010) Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB 12:509–522
McGlynn KA, London WT (2011) The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 15:223–243 vii-x
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Ozaki Y, Matsui H, Asou H, Nagamachi A, Aki D, Honda H et al (2012) Poly-ADP ribosylation of Miki by tankyrase-1 promotes centrosome maturation. Mol Cell 47:694–706
Chang P, Jacobson MK, Mitchison TJ (2004) Poly(ADP-ribose) is required for spindle assembly and structure. Nature 432:645–649
Asou H, Matsui H, Ozaki Y, Nagamachi A, Nakamura M, Aki D et al (2009) Identification of a common microdeletion cluster in 7q21.3 subband among patients with myeloid leukemia and myelodysplastic syndrome. Biochem Biophys Res Commun 383:245–251
Nigg EA (2002) Centrosome aberrations: cause or consequence of cancer progression? Nat Rev Cancer 2:815–825
Chung Moh M, Hoon Lee L, Shen S (2005) Cloning and characterization of hepaCAM, a novel Ig-like cell adhesion molecule suppressed in human hepatocellular carcinoma. J Hepatol 42:833–841
Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J et al (2010) Antibody validation. BioTechniques 48:197–209
Kondo Y (1985) Histologic features of hepatocellular carcinoma and allied disorders. Pathol Annu 20(Pt 2):405–430
Sugihara S, Nakashima O, Kojiro M, Majima Y, Tanaka M, Tanikawa K (1992) The morphologic transition in hepatocellular carcinoma. A comparison of the individual histologic features disclosed by ultrasound-guided fine-needle biopsy with those of autopsy. Cancer 70:1488–1492
Hidaka T, Hama S, Shrestha P, Saito T, Kajiwara Y, Yamasaki F et al (2009) The combination of low cytoplasmic and high nuclear expression of p27 predicts a better prognosis in high-grade astrocytoma. Anticancer Res 29:597–603
Matsuda Y, Yoshimura H, Ishiwata T, Sumiyoshi H, Matsushita A, Nakamura Y, et al (2016) Mitotic index and multipolar mitosis in routine histologic sections as prognostic markers of pancreatic cancers: a clinicopathological study. Pancreatology : official journal of the international association of Pancreatology16:127–132
Batistatou A (2004) Mitoses and cancer. Med Hypotheses 63(2):281
Gisselsson D, Hakanson U, Stoller P, Marti D, Jin Y, Rosengren AH et al (2008) When the genome plays dice: circumvention of the spindle assembly checkpoint and near-random chromosome segregation in multipolar cancer cell mitoses. PLoS One 3:e1871
Chan KS, Koh CG, Li HY (2012) Mitosis-targeted anti-cancer therapies: where they stand. Cell Death Dis 3:e411
Hallett RM, Huang C, Motazedian A, Auf der Mauer S, Pond GR, Hassell JA et al (2015) Treatment-induced cell cycle kinetics dictate tumor response to chemotherapy. Oncotarget 6:7040–7052
Weaver BA (2014) How Taxol/paclitaxel kills cancer cells. Mol Biol Cell 25:2677–2681
Acknowledgements
This work was supported by Pathology Department at Hospital Universitario de Araba-Txagorritxu.
Author information
Authors and Affiliations
Contributions
IFV made the diagnoses of hepatocellular carcinomas, carried out the TMA preparations together with ECU, and coordinated the study. Immunohistochemistry was carried out by FBGC. Data analyses were performed by JSJ, and JJA. LLG, ECU and IFV carried out and interpreted the staining, contributed to data analyses and drafted the manuscript. BG, IGM and LMQ provided technical support and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest in the research.
Rights and permissions
About this article
Cite this article
Fernández-Vega, I., Santos-Juanes, J., Camacho-Urkaray, E. et al. Miki (Mitotic Kinetics Regulator) Immunoexpression in Normal Liver, Cirrhotic Areas and Hepatocellular Carcinomas: a Preliminary Study with Clinical Relevance. Pathol. Oncol. Res. 26, 167–173 (2020). https://doi.org/10.1007/s12253-018-0387-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-0387-7