Abstract
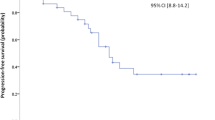
Everolimus is indicated for adults with metastatic renal cell carcinoma (mRCC) after failure of vascular endothelial growth factor receptor-tyrosine kinase inhibitors (TKI). Currently, the therapeutic applicability of EVE has been changing. Multicenter evaluation of efficacy and safety of everolimus in daily routine and definition of patient characteristics with favorable outcome. Data of 165 patients from 9 oncology institutes in Hungary were analyzed retrospectively. Everolimus therapy was used after one TKI in 10 mg starting dose. Physical and laboratory examinations and imaging tests were performed monthly and every 3 months, respectively. Median progression-free survival (PFS) was 5.4 months. Median overall survival (OS) was 16.2 months. PFS and OS results were more favorable in patients with ECOG 0–1 (pPFS = 0.033, pOS = 0.008) and after >9 months of TKI therapy (pPFS = 0.019, pOS = 0.045). Survival was longer in nonanemic patients with ECOG 0–1 than in anemic patients with ECOG 2–3, 30.9 and 7.7 months, respectively (p = 0.029). Dose reduction and treatment delay was required in 6.2% and 8.9% of patients, respectively. Common adverse events were exanthema, edema, stomatitis, anemia, and abnormal kidney functions and glucose levels. Results of this study show that everolimus is safe and efficacious in a real-world setting. Everyday practice showed that nonanemic patients with good performance status receiving TKI therapy for >9 months are favorable candidates for this treatment. Despite the efficiency of novel, registered drugs, everolimus still plays an important role during and after second-line therapy for mRCC when availability of modern remedies is limited.

Similar content being viewed by others
References
Afinitor EMEA Summary of Product Characteristics: http://www.ema.europa.eu/docs/hu_HU/document_library/EPAR_-_Product_Information/human/001038/WC500022814.pdf
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18:1926–1945
Eisen HJ, Tuzcu M, Dorent R et al (2003) Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant patients. N Engl J Med 349:847–858
Motzer RJ, Escudier B, Oudard S et al (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372:449–456
Yao JC, Shah MH, Ito T et al (2011) Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N Engl J Med 364:514–523
Yao JC, Fazio N, Singh S et al (2016) Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387:968–977
Baselga J, Campone M, Piccart M et al (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529
Motzer RJ, Escudier B, Oudard S et al (2010) Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 116:4256–4265
Calvo E, Escudier B, Motzer RJ et al (2011) Everolimus in metastatic renal cell carcinoma: subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 study. Eur J Cancer 48:333–339
NCCN Guideline. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
Escudier B, Porta C, Schmidinger M et al (2014) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:49–56
Ljungberg B, Bensalah K, Canfield S et al (2015) EAU Guidelines on Renal Cell Carcinoma: 2014 Update. Eur Urol 67:913–924
Motzer RJ, Escudier B, McDermott DF, el al (2015) Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 373:1803–1813
Choueiri TK, Escudier B, Powles T et al (2015) Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 373:1814–1823
Choueiri TK, Escudier B, Powles T et al (2016) Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17:917–927
Motzer RJ, Hutson TE, Glen H et al (2015) Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomized, phase 2, open-label, multicenter trial. Lancet Oncol 16:1473–1482
Escudier B, Porta C, Schmidinger M et al (2016) Renal Cell Carcinoma: ESMO Clinical Practice Guidelines. Ann Oncol 27:58–68
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–214
Common Terminology Criteria for Adverse Events, version 3.0 issued by National Cancer Institute EORTC, 09. 08 (2006) http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
ECOG performance status. http://ecog.dfci.harvard.edu/general/perf_stat.html
Rini BI, Flaherty K (2008) Clinical effect and future considerations for molecularly-targeted therapy in renal cancer. Uro Oncol Semin Orig Investig 26:543–549
Grünwald V, Karakiewicz PI, Bavbek SE et al (2012) An international expanded-access programme of everolimus: addressing safety and efficacy in patients with metastatic renal cell carcinoma who progress after initial vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapy. Eur J Cancer 48:324–332
Calvo E, Ravaud A, Bellmunt J (2013) What is the optimal therapy for patients with metastatic renal cell carcinoma who progress on an initial VEGFr-TKI? Cancer Treat. Rev. 39:366–374
Bergmann L, Kube U, Doehn C et al (2015) Everolimus in metastatic renal cell carcinoma after failure of initial anti–VEGF therapy: final results of a noninterventional study. BMC Cancer 15:303
Motzer RJ, Bacik J, Schwartz LH et al (2004) Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 22:454–463
Acknowledgments
We express our thanks to the doctors for their work and for the data they made available for this study: Csilla András, Krisztina Bíró, Anita Bokor, Zsófia Dankovics, Gergely Dombóvári, Andrea Gonda, Fruzsina Gyergyai, Attila Huszár, Balázs Juhász, Erika Kövér, Zsófia Küronya, Zsuzsanna Miszlai, Krisztián Nagyiványi, Hajnalka Németh, Ágota Petrányi, Tibor Solymosi, Éva Szilágyi, Éva Szekanecz, Judit Tóth, Miklós Wenczl and Judit Zsálek, and all members of their institutes who contributed to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Anikó Maráz has received honoraria from Bayer, Bristol-Myers Squibb, and has served on advisory boards for Novartis.
András Csejtei has served on advisory boards for Novartis and Pfizer.
Judit Kocsis has received honoraria from Bayer and served as a member of advisory board: Novartis, Bristol-Myers Squibb and Pfizer.
Miklós Szűcs has received honoraria from Bayer, Novartis, Bristol-Myers Squibb and has served on advisory boards for Novartis and Pfizer.
Zsuzsanna Kahán has no actual or potential conflicts of interest to report.
György Bodoky has received honoraria from Bayer, Bristol-Myers Squibb and Pfizer and has served on advisory boards for Novartis and Pfizer.
Magdolna Dank has received honoraria from Bayer, Novartis and Pfizer and has served on advisory boards for Novartis and Pfizer.
László Mangel has received honoraria from Pfizer and has served on advisory boards for Novartis and Pfizer.
János Révész has served on advisory boards for Novartis and Pfizer.
Zoltán Varga has no actual or potential conflicts of interest to report.
Lajos Géczi has received honoraria from Bayer, Novartis, Bristol-Myers Squibb and Pfizer and has served on advisory boards for Bristol-Myers Squibb, Novartis and Pfizer.
Ethical Approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study no formal consent is required.
Support
This was a non-sponsored study.
Additional information
The authors are solely responsible for writing the manuscript and the decision to submit the manuscript for publication.
Rights and permissions
About this article
Cite this article
Maráz, A., Csejtei, A., Kocsis, J. et al. Assessment of the Role of Everolimus Therapy in Patients with Renal Cell Carcinoma Based on Daily Routine and Recent Research Results. Pathol. Oncol. Res. 25, 149–156 (2019). https://doi.org/10.1007/s12253-017-0317-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-017-0317-0