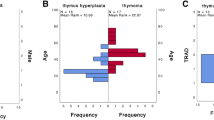
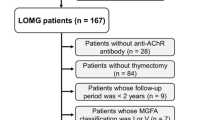
Abstract
Thymectomy is routinely carried out in patients with myasthenia gravis (MG) and thymomas. However, there is still a dispute as to whether MG patients with thymic hyperplasia should undergo thymectomy. We aimed to investigate the pathological findings in the thymus in patients with co-existing MG and thymic hyperplasia or thymomas treated with thymectomy, as well as effects of immunosuppression. Thirty-three patients with MG were selected and grouped accordingly: patients with no thymic abnormalities, patients with thymic hyperplasia, and patients with thymomas. All patients were treated with methylprednisolone alongside immunosuppression. A separate cohort of 24 MG patients with thymic hyperplasia or thymomas and treated with thymectomy were selected. As controls, 5 patients with thymomas or thymic carcinoma without MG were selected. Expression of CD5, extracellular regulated protein kinases1/2 mitogen activated protein kinase (ERK1/2MAPKs) and CD95 ligand (FasL) in the thymus was examined. Methylprednisolone and immunosuppressive therapy are highly effective in MG patients with normal thymus tissue and MG patients with thymic hyperplasia compared to MG patients with thymomas alone. CD5 expression was highest in MG patients with thymic hyperplasia, correlating with expression of ERK1/2MAPKs. FasL expression was similar across all groups. Thymomas may be distinguished from thymic hyperplasia by expression of CD5 and ERK1/2MAPKs. Thymectomy is the preferred treatment for MG patients with thymomas but may not be necessary in MG patients with thymic hyperplasia who are treated with immunosuppressive therapy.


Similar content being viewed by others
References
Vincent A (2002) Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol 2(10):797–804
Moser B, Bekos C, Zimprich F, Nickl S, Klepetko W, Ankersmit J (2012) The receptor for advanced glycation end products and its ligands in patients with myasthenia gravis. Biochem Biophys Res Commun 420(1):96–101
Berrih-Aknin S, Le Panse R (2014) Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun 52:90–100
Gronseth GS, Barohn R (2000) Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review). Report from the quality standards subcommittee of the American academy of neurology. Neurology 55:7–15
Bak V, Spalek P, Petrovajova T, Vichova B, Oravsky M, Luha J, Schnorrer M (2013) Thymic tumours associated with Myasthenia gravis: a long term observation study of operated patients. Bratisl Lek Listy 114(8):464–468
Cavalcante P, Le Panse R, Berrih-Aknin S, Maggi L, Antozzi C, Baggi F, Bernasconi P, Mantegazza R (2011) The thymus in myasthenia gravis: site of "innate autoimmunity"? Muscle Nerve 44(4):467–484
Geuder KI, Marx A, Witzemann V, Schalke B, Kirchner T, Müller-Hermelink HK (1992) Genomic organization and lack of transcription of the nicotinic acetylcholine receptor subunit genes in myasthenia gravis-associated thymoma. Lab Investig 66(4):452–458
Sandri A, Cusumano G, Lococo F et al (2014) Long-term results after treatment for recurrent thymoma: a multicenter analysis. J Thorac Oncol 9(12):1796–1804
De Meel RH, Lipka AF, Van Zwet EW, Niks EH, Verschuuren JJ (2015) Prognostic factors for exacerbations and emergency treatments in myasthenia gravis. J Neuroimmunol 282:123–125
Safieddine N, Liu G, Cuningham K et al (2014) Prognostic factors for cure, recurrence and long-term survival after surgical resection of thymoma. J Thorac Oncol 9(7):1018–1022
Uzawa A, Kawaguchi N, Kanai T et al (2015) Two-year outcome of thymectomy in non-thymomatous late-onset myasthenia gravis. J Neurol 262(4):1019–1023
Azzam HS, DeJarnette JB, Huang K et al (2001) Fine tuning of TCR signaling by CD5. J Immunol 166(9):5464–5472
Tarakhovsky A, Kanner SB, Hombach J et al (1995) A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science 269(5223):535–537
Ragheb S, Lisak RP (1992) Effect of clinical status and treatment on the frequency of CD5+ B cells in patients with myasthenia gravis. Neurology 42(5):1076–1080
Dalloul A (2009) CD5: a safeguard against autoimmunity and a shield for cancer cells. Autoimmun Rev 8(4):349–353
Ragheb S, Lisak RP (1990) The frequency of CD5+ B lymphocytes in the peripheral blood of patients with myasthenia gravis. Neurology 40(7):1120–1124
Colombara M, Antonini V, Riviera AP et al (2005) Constitutive activation of p38 and ERK1/2 MAPKs in epithelial cells of myasthenic thymus leads to IL-6 and RANTES overexpression: effects on survival and migration of peripheral T and B cells. J Immunol 175(10):7021–7028
Friedlein G, El Hage F, Vergnon I et al (2007) Human CD5 protects circulating tumor antigen-specific CTL from tumor-mediated activation-induced cell death. J Immunol 178(11):6821–6827
Lundy SK, Fox DA (2009) Reduced Fas ligand-expressing splenic CD5+ B lymphocytes in severe collagen-induced arthritis. Arthritis Res Ther 11(4):R128
Aruna BV, Ben-David H, Sela M et al (2006) A dual altered peptide ligand down-regulates myasthenogenic T cell responses and reverses experimental autoimmune myasthenia gravis via up-regulation of Fas–FasL-mediated apoptosis. Immunology 118(3):413–424
Zhang J, Gao JX, Salojin K et al (2000) Regulation of Fas ligand expression during activation-induced cell death in T cells by p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase. Exp Med 191:1017–1030
Krammer PH (2000) CD95's deadly mission in the immune system. Nature 407:789–495
Davidson WF, Haudenschild C, Kwon J, Williams MS (2002) T cell receptor ligation triggers novel nonapoptotic cell death pathways that are Fas-independent or Fas-dependent. J Immunol 169:6218–6230
Jaretzki A 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, Sanders DB (2000) Myasthenia gravis: recommendations for clinical research standards. J Ann Thorac Surg 70(1):327–334
Osserman KE, Genkins G (1961) Studies in myasthenia gravis. N Y State J Med 61:2076–2085
Murai H (2015) Myasthenia gravis: epidemiology and prognosis. Nihon Rinsho 73(Suppl 7):472–476
Utsugisawa K, Nagane Y, Suzuki S, Suzuki N (2012) Directions for use of corticosteroids and calcineurin inhibitors against generalized myasthenia gravis: therapeutic strategies that can lead to early improvements and veer away from high-dose oral corticosteroids. Clin Neuro l52(11):1047–1050
Kim JY, Park KD, Richman DP (2011) Treatment of myasthenia gravis based on its immunopathogenesis. J Clin Neurol 7(4):173–183
Diaz A, Black E, Dunning J (2014) Is thymectomy in non-thymomatous myasthenia gravis of any benefit? Interact Cardiovasc Thorac Surg 18(3):381–389
Le Panse R, Bismuth J, Cizeron-Clairac G et al (2010) Thymic remodeling associated with hyperplasia in myasthenia gravis. Autoimmunity 43(5–6):401–412
Yi Q, Ahlberg R, Pirskanen R, Lefvert AK (1992) Levels of CD5+ B lymphocytes do not differ between patients with myasthenia gravis and healthy individuals. Neurology 42(5):1081–1084
Aruna BV, Ben-David H, Sela M, Mozes E (2006) A dual altered peptide ligand down-regulates myasthenogenic T cell responses and reverses experimental autoimmune myasthenia gravis via up-regulation of Fas-FasL-mediated apoptosis. Immunology 118(3):413–424
Shin HJ, Katz RL (1998) Thymic neoplasia as represented by fine needle aspiration biopsy of anterior mediastinal masses. A practical approach to the differential diagnosis. Acta Cyto l42(4):855–864
Li H, Wang DL, Liu XW, Geng ZJ, Xie CM (2013) Computed tomography characterization of neuroendocrine tumors of the thymus can aid identification and treatment. Acta Radio l54(2):175–180
Acknowledgements
We thank Dr. Lee-Ming Kow at Rockefeller University and Dr. Ding-Guo Shen at Xi’an Gaoxin Hospital for their valuable advice in manuscript preparation.
Author Contributions
Dr. Ping Chen, Ying-Peng Wang, Dan-lei Mou: study concept, analysis, literature review, and initial draft of paper; Dr. Zheng-Yi Li, Qiu-Min Qu, Ting Wang, Li-Hua Chen: acquisition of data for literature search, clinical scoring and analysis; Dr.Hong-Yan Wang, Yuan Deng, Xiao-Feng Li: tissue processing, immunohistochemistry experiments and analysis; Dr. Xian-hao Xu, Gang Zhao: critical revision of manuscript for intellectual content and clarity.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interests
None to declare.
Study Funding
National Natural Science Foundation of China 81201294.
47th Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, 2nd degree.
Disclosure
This article has no disclosures relevant to the manuscript.
Additional information
Ping Chen, Ying-Peng Wang, Dan-lei Mou, Xian-Hao Xu and Gang Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, P., Wang, YP., Mou, Dl. et al. Pathological Findings in Myasthenia Gravis Patients with Thymic Hyperplasia and Thymoma. Pathol. Oncol. Res. 24, 67–74 (2018). https://doi.org/10.1007/s12253-017-0213-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-017-0213-7