Abstract
Purpose
Gliclazide (GLC)-loaded Aerosil 380 solid dispersion (GA-SD)-based tablets with co-processed excipient composites were formulated to critically evaluate the physicochemical performance of the resulting tablets with enhanced drug release.
Methods
GA-SD was prepared using the solvent evaporation method with a 1:1 weight ratio based on a previously published report, and its drug release patterns were evaluated. Processed excipient composites, such as lactose-starch-povidone (LSP) and lactose-starch-povidone-sodium starch glycolate (LSPS), were prepared via a coprocessing strategy and evaluated for their ability to perform specific functions. At predetermined combination levels, aqueous dispersions of primary excipients were physically agglomerated at a controlled temperature below the gelatinization temperature (55 °C) before drying at 60 °C for 48 h. GA-SD and co-processed excipients (LSP and LSPS) were utilized to produce tablet batches GAC1 to GAC8 (Gliclazide-Aerosil 380–co-processed excipients, GAC) by direct compression. Through rigorous testing of tablet batches, the physicochemical properties of the resulting formulations were analyzed and compared to those of leading marketed formulations (MFs). FTIR studies were also conducted to detect drug-excipient interactions in the tablet formulations. The release mechanism of the GLC was determined by studying the dissolution process with various kinetic models. The GAC tablets were subjected to 40 °C/75% RH for 3 months to assess stability.
Results
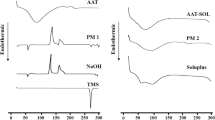
All tablet formulations of GA-SD containing co-processed excipients met the weight, friability, disintegration time, mechanical strength, and homogeneity requirements. There was significantly more GLC released from the GAC formulations (p < 0.05) at each time point when the formulations were exposed to water than when the formulations were exposed to MFs. In vitro, testing revealed that the GAC5 to GAC8 formulations were the most efficient due to the presence of the superdisintegrant in the LSPS composite, which may be a contributing factor to the improvement in the dissolution rate by GA-SD. FTIR analysis revealed no notable chemical interactions between GLC and the excipients in the solid state. The Korsmeyer-Peppas model was the best-fit kinetic model, indicating that diffusion is the predominant mechanism of GLC dissolution. According to the commercial standards, the GAC tablets maintained an acceptable hardness, disintegration time, and drug content during the stability studies. Additionally, no significant changes in release profiles were observed in the selected batches (p < 0.05).
Conclusion
Compared with currently marketed formulations (MFs), GA-SD tablet formulations with co-processed excipients significantly improved the physicochemical properties, including the drug release rate. These findings could lead to the development of more effective and efficient tablet solid dosage forms of drugs with low water solubility, and co-processed excipients could be utilized as a more effective alternative to direct compression materials in tablet formulations.




Similar content being viewed by others
Availability of Data and Material
All the data generated or analyzed during this study has been included in the published article.
Code Availability
NA.
References
Rayaprolu BM, Strawser JJ, Anyarambhatla G. Excipients in parenteral formulations: selection considerations and effective utilization with small molecules and biologics. Drug Dev Ind Pharm. 2018. https://doi.org/10.1080/03639045.2018.1483392.
Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nat Biomed Eng. 2021. https://doi.org/10.1038/s41551-021-00698-w.
Martin C, Jiri D. Advances in dissolution instrumentation and their practical applications. Drug Dev Ind Pharm. 2014. https://doi.org/10.3109/03639045.2013.841184.
Kulkarni MC, Kolhe SV. Formulation development and evaluation of atorvastatin calcium tablets using co-processed excipients. Int J Pharm Sci Rev Res. 2016;36(1):217–22.
Atneriya U, Kapoor D, Sainy J, Maheshwari R. In vitro profiling of fenofibrate solid dispersion mediated tablet formulation to treat high blood cholesterol. Annales Pharma Fran. 2023. https://doi.org/10.1016/j.pharma.2022.08.009.
Sareen S, Mathew G, Joseph L. Improvement in solubility of poorly water-soluble drugs by solid dispersion. Int J Pharm Investig. 2012. https://doi.org/10.4103/2230-973X.96921.
Takeuchi H, Nagira S, Yamamoto H, Kawashima Y. Solid dispersion particles of amorphous indomethacin with fine porous silica particles by using spray-drying method. Int J Pharm. 2005. https://doi.org/10.1016/j.ijpharm.2004.12.019.
Choi JS, Lee SE, Jang WS, Byeon JC, Park JS. Tadalafil solid dispersion formulations based on PVP/VA S-630: Improving oral bioavailability in rats. Eur J Pharm Sci. 2017;106:152–8. https://doi.org/10.1016/j.ejps.2017.05.065.
Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J of Pharm Sci. 1971. https://doi.org/10.1002/jps.2600600902.
Zhao J, Koo O, Pan D, Wu Y, Morkhade D, Rana S, Saha P, Marin A. The impact of disintegrant type, surfactant, and API properties on the processability and performance of roller compacted formulations of acetaminophen and aspirin. AAPS J. 2017. https://doi.org/10.1208/s12248-017-0104-6.
Jain KK. An overview of drug delivery systems. In: Jain K, editor. Drug delivery systems. Methods in molecular biology. New York, NY: Humana; 2020. https://doi.org/10.1007/978-1-4939-9798-5_1.
Armstrong NA. Tablet manufacture by direct compression. In: Swarbrick J, editor. Encyclopaedia of Pharmaceutical Technology. 3rd ed. USA: Informa Healthcare; 2007. p. 3673–83.
Alderborn G. Tablets and compaction. In: Aulton ME, Taylor KM, editors. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines. 4th ed. London: Elsevier Ltd; 2013. p. 504–49.
Augsburger LL, Zellhofer MJ. Tablet formulation. In: Encyclopaedia of pharmaceutical technology. 3rd ed. USA: Informa Healthcare; 2007. p. 3641–52.
Thoorens G, Krier F, Leclercq B, Carlin B, Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment- a review. Int J Pharm. 2014;473:64–72.
Li Z, Zhao L, Lin X, Shen L, Feng Y. Direct compaction: an update of materials, trouble-shooting, and application. Int J Pharm. 2017. https://doi.org/10.1016/j.ijpharm.2017.07.035.
Gonnissen Y, Verhoeven E, Peeters E, Remon J, Vervaet C. Coprocessing via spray drying as a formulation platform to improve the compactibility of various drugs. Eur J Pharm and Biopharm. 2008;69:320–34.
Apeji YE, Aluga D, Olayemi OJ, Oparaeche C, Anyebe SN, Gamlen MJ, Oyi AR. Comparative analysis of co-processed starches prepared by three different methods. British J Pham. 2017. https://doi.org/10.5920/bjpharm.2017.08.
van der Merwe J, Steenekamp J, Steyn D, Hamman J. The role of functional excipients in solid oral dosage forms to overcome poor drug dissolution and bioavailability. Pharmaceutics. 2020. https://doi.org/10.3390/pharmaceutics12050393.
Crowley PJ, Martini LG. Formulation design: new drugs from old. Drug Discov Today Ther Strateg. 2004. https://doi.org/10.1016/j.ddstr.2004.11.020.
Guth F, Schiffter HA, Kolter K. Novel excipients-from concept to launch. Chim Oggi Chem Today. 2013;31:78–81.
Gohel MC, Jogani PD. A review of co-processed directly compressible excipients. Journal of Pharmacy & Pharmaceutical Sciences: a publication of the Canadian Society for Pharmaceutical Sci. 2005;8(1):76–93.
Gupta P, Nachaegari SK, Bansal AK. Improved excipient functionality by coprocessing. In: Katdare A, Chaubal M, editors. Excipient development for pharmaceutical, biotechnology and drug delivery systems. CRC Press; 2006. p. 109–26. https://doi.org/10.1201/9781420004137.
Desai U, Shavan R, Mhatre P, Chinchole R. A review: coprocessed excipients. Int J Pharm Sci Rev Research. 2012;12(2):93–105.
Rojas J, Buckner I, Kumar V. Co-processed excipients with enhanced direct compression functionality for improved tableting performance. Drug Dev Ind Pharm. 2012;38(10):1159–70.
Salim I, Kehinde OA, Abdulsamad A, Khalid GM, Gwarzo MS. Physicomechanical behavior of novel directly compressible starch-MCC-povidone composites and their application in ascorbic acid tablet formulation. British J Pharm. 2018. https://doi.org/10.5920/bjpharm.2018.03.
Saha S, Shahiwala AF. Multifunctional coprocessed excipients for improved tabletting performance Multifunctional coprocessed excipients for improved tabletting performance. Drug Delivery. 2009. https://doi.org/10.1517/17425240802708978.
Builders PF, Bonaventure AM, Tiwalade A, Okpako LC, Attama AA. Novel multifunctional pharmaceutical excipients derived from microcrystalline cellulose-starch microparticulate composites prepared by compatibilized reactive polymer blending. Int J Pharm. 2010;388:159–67.
Ozkan Y, Atay T, Dikmen N, Aboul-Enein HY. Improvement of water solubility and in vitro dissolution rate of gliclazide by complexation with β-cyclodextrin. Pharm Acta Helv. 2000. https://doi.org/10.1016/s0031-6865(99)00063-1.
Harrower AD. Comparison of efficacy, secondary failure rate and complications of sulfonylurea. J Diabetes Its Complicat. 1994. https://doi.org/10.1016/1056-8727(94)90044-2.
Palmer KJ, Brogden RN. Gliclazide, an update of its pharmacological properties and therapeutic efficacy in NIDDM. Drugs. 1993. https://doi.org/10.2165/00003495-199346010-00007.
Alkhamis KA, Allaboun H, Al-Momani WY. Study of the solubilization of gliclazide by aqueous micellar solutions. J Pharm Sci. 2003. https://doi.org/10.1002/jps.10350.
Varshosaz J, Talari R, Mostafavi SA, Nokhodchi A. Dissolution enhancement of gliclazide using in situ micronization by solvent change method. Pow Tech. 2008. https://doi.org/10.1016/j.powtec.2008.02.018.
Saharan VA, Choudhury P. Dissolution rate enhancement of gliclazide by ordered mixing. Acta Pharm. 2011. https://doi.org/10.2478/v10007-011-0021-7.
Biswal S, Sahoo J, Murthy PN, Giradkar RP, Avari JG. Enhancement of dissolution rate of gliclazide using solid dispersions with polyethylene glycol 6000. AAPS PharmSciTech. 2008. https://doi.org/10.1208/s12249-008-9079-z.
Paul S, Islam MN, Ali MA, Barman RK, Wahed MII, Rahman BM. Improvement of dissolution rate of gliclazide using solid dispersions with aerosil 380 and its effect on alloxan-induced diabetic rats. Pharmacology and Pharmacy. 2019. https://doi.org/10.4236/pp.2019.108030.
Biswal S, Sahoo J, Murthy PN. Physicochemical properties of solid dispersions of gliclazide in polyvinylpyrrolidone K90. AAPS PharmSciTech. 2009. https://doi.org/10.1208/s12249-009-9212-7.
Febriyenti F, Rahmi S, Halim A. Study of gliclazide solid dispersion systems using PVP k-30 and PEG 6000 by solvent method. J Pharm Bioallied Sci. 2019. https://doi.org/10.4103/jpbs.JPBS_87_18.
Wang L, De Cui F, Sunada H. Preparation and evaluation of solid dispersions of nitrendipine prepared with fine silica particles using the melt-mixing method. Chem Pharm Bull. 2006. https://doi.org/10.1248/cpb.54.37.
Varma MM, Kumar PS. Formulation and evaluation of gliclazide tablets containing PVP-K30 and Hydroxy propyl-β-cyclodextrin solid dispersion. Int J Pharm Sci Nanotech. 2012;5(2):1706–19.
Fuley MJ, Parisar S. Preparation and characterization of gliclazide-polyethylene glycol 4000 solid dispersions. Acta Pharm. 2009;59:57–65.
Dukhan AAM, Amalina N, Oo MK, Sengupta P, Doolaanea AAM, Aljapairai KAS, Chatterjee B. Formulation of dispersed gliclazide powder in polyethylene glycol–polyvinyl caprolactam–polyvinyl acetate grafted copolymer carrier for capsulation and improved dissolution. Indian J Pharm Educ Res. 2018. https://doi.org/10.5530/ijper.52.4s.100.
Mooranian A, Negrulj R, Mathavan S, et al. Stability and release kinetics of an advanced gliclazide-cholic acid formulation: the use of artificial-cell microencapsulation in slow release targeted oral delivery of antidiabetics. J Pharm Innov. 2014. https://doi.org/10.1007/s12247-014-9182-5.
Bala M, Priya VK, Murthy T. Development of discriminative dissolution media for marketed gliclazide modified-release tablets. Dissolution Technol. 2012;19:38–42. https://doi.org/10.14227/DT190212P38.
Ibrahim SK, Nada A, Abdel-Azim Z. Physicochemical characterization of gliclazide–macrogol solid dispersion and tablets based on optimized dispersion. Drug Dev Ind Pharm. 2010. https://doi.org/10.3109/03639040903578734.
OlowosuluA K, Isah AB, Ibrahim MA. Physicochemical characterization and tableting properties of starch 955 and starch 9010 - new co-processed starch-based excipients. Nigerian J Pharm Sci. 2011;10(2):57–69.
Singh SY, Salwa, Shirodkar RK, et al. Enhancement in dissolution rate of atorvastatin trihydrate calcium by formulating its porous tablet using sublimation technique. J Pharm Innov. 2020;15:498–520. https://doi.org/10.1007/s12247-019-09397-1.
United States Pharmacopeial Convention. The United States Pharmacopeia: USP 24; the National Formulary: NF 19: Official from January 1 2000. Rockville, MD: United States Pharmacopeial Convention; 1999.
Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2012. https://doi.org/10.1016/j.addr.2012.09.028.
Parezanović GŠ, Lalić-Popović M, Goločorbin-Kon S, Todorović N, Pavlović N, Mikov M. In vitro comparative quality evaluation of non-expired and 10 years-expired lamotrigine immediate-release tablet formulations-pilot study. Dissolution Technol. 2020;27:14–20. https://doi.org/10.14227/DT270120P14.
Almotairi N, Mahrous GM, Al-suwayeh S, Kazi M. Design and optimization of lornoxicam dispersible tablets using quality by design (QbD) approach. Pharmaceuticals. 2022. https://doi.org/10.3390/ph15121463.
Mircioiu C, Voicu V, Anuta V, Tudose A, Celia C, Paolino D, Fresta M, Sandulovici R, Mircioiu I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics. 2019. https://doi.org/10.3390/pharmaceutics11030140.
Diaz DA, Colgan ST, Langer CS, Bandi NT, Likar MD, Van Alstine L. Dissolution similarity requirements: how similar or dissimilar are the global regulatory expectations? AAPS J. 2016. https://doi.org/10.1208/s12248-015-9830-9.
Mahajan HS, Girnar GA, Nerkar PP. Dissolution and bioavailability enhancement of gliclazide by surface solid dispersion using spray drying technique. Ind J Novel Drug Deliv. 2012;4:115–24.
Baghel S, Cathcart H, O’Reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J Pharm Sci. 2016. https://doi.org/10.1016/j.xphs.2015.10.008.
Dharna A, Singh N, Sandeep A. Solid dispersions: a review on drug delivery system and solubility enhancement. Int J Pharm Sci Res. 2013;4(6):2094–105.
Onuki Y, Kosugi A, Hamaguchi M, Marumo Y, Kumada S, Hirai D, Ikeda J, Hayashi Y. A comparative study of disintegration actions of various disintegrants using Kohonen’s self-organizing maps. J Drug Deliv Sci Technol. 2018. https://doi.org/10.1016/j.jddst.2017.10.002.
Hebbink GA, Dickhoff BHJ. Chapter 5 - Application of lactose in the pharmaceutical industry. In: Paques M, Lindner C, editors. Lactose. Academic Press; 2019. p. 175–229. https://doi.org/10.1016/B978-0-12-811720-0.00005-2.
Carlson JA, Mann HJ, Canafax DM. Effect of pH on disintegration and dissolution of ketoconazole tablets. Am J Hosp Pharm. 1983;40:1334–8.
Mohapatra S, Kar RK, Sahoo SK. Goodness of fit model dependent approaches of controlled release matrix tablets of zidovudine. Indian J Pharm Educ Res. 2016. https://doi.org/10.5530/ijper.50.1.18.
Bruschi ML, editor. 5 - Mathematical models of drug release. In: Strategies to modify drug release from pharmaceutical systems. Woodhead Publishing; 2015. p. 63–86. https://doi.org/10.1016/B978-0-08-100092-2.00005-9.
Acknowledgements
The authors thank the Faculty of Science at the University of Rajshahi for partially funding this research. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R301), King Saud University, Riyadh, Saudi Arabia. The authors are grateful for the gliclazide and magnesium stearate sample gift from Square Pharma Ltd. (Bangladesh). The authors thank the Central Science Laboratory of the University of Rajshahi, Bangladesh, for conducting the FTIR analysis.
Funding
Partial financial support was received from the Dean of the Faculty of Science at the University of Rajshahi, Bangladesh. It is also supported by RSP2024R301, King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization: Md Bytul Mokaddesur Rahman; methodology: Israt Zerin Alam, Jakia Sultana; formal analysis and investigation: Israt Zerin Alam, Jakia Sultana, Mohsin Kazi; writing—original draft preparation: Israt Zerin Alam, Md Bytul Mokaddesur Rahman; writing—review and editing: Md Bytul Mokaddesur Rahman, Mohsin Kazi, Mohammad N. Uddin; supervision: Md Bytul Mokaddesur Rahman.
Corresponding authors
Ethics declarations
Ethics Approval
NA
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alam, I.Z., Sultana, J., Kazi, M. et al. In Vitro Profiling of Gliclazide-Loaded Aerosil 380 Solid Dispersion–Based Tablets with Co-Processed Excipients. J Pharm Innov 19, 17 (2024). https://doi.org/10.1007/s12247-024-09817-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s12247-024-09817-x