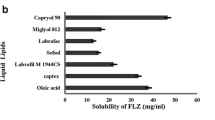
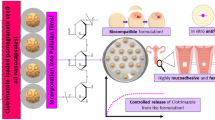
Abstract
Purpose
This study aimed to produce lipid complex-ITR (CL-ITR) to enhance the solubility of ITR which is then made into an in situ vaginal gel to maximize the local therapeutic effect of vulvovaginal candidiasis.
Methods
The CL-ITR was prepared by mixing ITR with Phospholipon 90G® (P90G) in different concentrations with solvent-evaporated methods. CL-ITR optimization was carried out by determining the solubility of ITR in CL-ITR and then characterized through FTIR, DSC, SEM, and profile release of ITR. Furthermore, the optimal CL-ITR formula was mixed into the gel base using Poloxamer (PF127 and PF68) and sodium alginate (SA) as gelling agents with various concentrations. The gel characteristics were then determined through Tsol-gel, pH, viscosity, time and mucoadhesive strength, and stability tests. Ex vivo evaluation was carried out using the porcine’s model to determine ITR that was permeated and retained on the vaginal mucosa and antifungal activity assay.
Results
The F5 with an ITR:P90G concentration of 1:5 is an optimized CL-ITR that is soluble ITR at 1275 ± 10.24 mg/mL. The G3 is the formula gel that retained the highest ITR, namely 4.72 ± 0.06 mg, and it had the lowest permeation of 1.90 ± 0.02 mg at 24 h. Furthermore, the time-killing test showed that G3 is the superlative formula against C. albicans, namely 3.58 ± 0.03 CFU/mL at 24 h.
Conclusion
ITR was successfully administered vaginally in the form of an in situ gel and had desirable values in every evaluation that was performed. Therefore, it can be an alternative to improve the effectiveness of ITR therapy for the treatment of vulvovaginal candidiasis.





Similar content being viewed by others
References
Akhtar S, Masood S, Tabassum S, Rizvi DA. Efficacy of itraconazole versus fluconazole in vaginal candidiasis. J Pakistan Med Assoc. 2012;62.
Botros SR, Hussein AK, Mansour HF. A novel nanoemulsion intermediate gel as a promising approach for delivery of itraconazole: design, in vitro and ex vivo appraisal. AAPS PharmSciTech. 2020;21:12–4. https://doi.org/10.1208/s12249-020-01830-w.
Bagavatula H, Lankalapalli S, Tenneti VVK, Beeraka NMR, Bulusu BT. Comparative studies on solubility and dissolution enhancement of different itraconazole salts and their complexes. Adv Pharmacol Pharm. 2014;2:85–95. https://doi.org/10.13189/app.2014.020601.
Kalia K, Poddar M. Solid dispersions: an approach towards enhancing dissolution rate. Int J Pharm Pharm Sci. 2011;3(9–19).
Permana AD, Utomo E, Pratama MR, Amir MuhN, Anjani QK, Mardikasari SA, et al. Bioadhesive-thermosensitive in situ vaginal gel of the gel flake-solid dispersion of itraconazole for enhanced antifungal activity in the treatment of vaginal candidiasis. ACS Appl Mater Interfaces. 2021. https://doi.org/10.1021/acsami.1c03422.
Kumar N, Shishu, Bansal G, Kumar S, Jana AK. Ditosylate salt of itraconazole and dissolution enhancement using cyclodextrins. AAPS PharmSciTech. 2012;13:863–74. https://doi.org/10.1208/s12249-012-9804-5.
Vasilev NA, Surov AO, Voronin AP, Drozd KV, Perlovich GL. Novel cocrystals of itraconazole: insights from phase diagrams, formation thermodynamics and solubility. Int J Pharm. 2021;599. https://doi.org/10.1016/j.ijpharm.2021.120441.
Kathpalia H, Salunkhe S, Juvekar S. Formulation of gastroretentive sustained release floating in situ gelling drug delivery system of solubility enhanced curcumin-soy lecithin complex. J Drug Deliv Sci Technol. 2019;53. https://doi.org/10.1016/j.jddst.2019.101205.
Abdellatif MM, Khalil IA, Elakkad YE, Eliwa HA, Samir TM, Al-Mokaddem AK. Formulation and characterization of sertaconazole nitrate mucoadhesive liposomes for vaginal candidiasis. Int J Nanomedicine. 2020;15:4079–90. https://doi.org/10.2147/IJN.S250960.
Wirantari N, Hidayati AN. The role of Lactobacillus in managing endogenous female tract infections. 2018. https://doi.org/10.33820/mdvi.v45i2.22.
Srikrishna S, Cardozo L. The vagina as a route for drug delivery: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2013;24:537–43. https://doi.org/10.1007/s00192-012-2009-3.
Sosa L, Calpena AC, Silva-Abreu M, Espinoza LC, Rincón M, Bozal N, et al. Thermoreversible gel-loaded amphotericin B for the treatment of dermal and vaginal candidiasis. Pharmaceutics. 2019;11:312. https://doi.org/10.3390/pharmaceutics11070312.
Mohammed NN, Pandey P, Khan NS, Elokely KM, Liu H, Doerksen RJ, et al. Clotrimazole–cyclodextrin based approach for the management and treatment of Candidiasis – a formulation and chemistry-based evaluation. Pharm Dev Technol. 2016;21:619–29. https://doi.org/10.3109/10837450.2015.1041041.
Fenny S, Safitri I. Overview: application of Carbopol 940 in Gel. 2021. https://doi.org/10.2991/ahsr.k.210127.018.
Khan J, Alexander A, Ajazuddin, Saraf S, Saraf S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J Controlled Release. 2013;168:50–60. https://doi.org/10.1016/j.jconrel.2013.02.025.
Cristiano MC, Froiio F, Mancuso A, De Gaetano F, Ventura CA, Fresta M, et al. The Rheolaser Master™ and Kinexus Rotational Rheometer® to evaluate the influence of topical drug delivery systems on rheological features of topical poloxamer gel. Molecules. 2020;25:1979. https://doi.org/10.3390/molecules25081979.
Puscaselu R, Gutt G, Amariei S. The use of edible films based on sodium alginate in meat product packaging: an eco-friendly alternative to conventional plastic materials. Coatings. 2020;10. https://doi.org/10.3390/coatings10020166.
Enggi CK, Isa HT, Sulistiawati S, Ardika KAR, Wijaya S, Asri RM, et al. Development of thermosensitive and mucoadhesive gels of cabotegravir for enhanced permeation and retention profiles in vaginal tissue: a proof of concept study. Int J Pharm. 2021;609:121182. https://doi.org/10.1016/j.ijpharm.2021.121182.
Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–5. https://doi.org/10.1016/S0010-7824(99)00010-4.
Permana AD, McCrudden MT, Donnelly RF. Enhanced intradermal delivery of nanosuspensions of antifilariasis drugs using dissolving microneedles: a proof of concept study. Pharmaceutics. 2019;11:346. https://doi.org/10.3390/pharmaceutics11070346.
WHO. Laboratory manual for diagnosis of fungal opportunistic infections in HIV/AIDS patients. 2009.
Güven UM, Berkman MS, Şenel B, Yazan Y. Development and in vitro/in vivo evaluation of thermo-sensitive in situ gelling systems for ocular allergy. Braz J Pharm Sci. 2019;55. https://doi.org/10.1590/s2175-97902019000117511.
Dantas MGB, Reis SAGB, Damasceno CMD, Rolim LA, Rolim-Neto PJ, Carvalho FO, et al. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Sci World J. 2016;2016:1–4. https://doi.org/10.1155/2016/7394685.
Fahrurroji A, Wijianto B, Styawan A. Formulasi dan Evaluasi Hidrogel Mukoadhesif Metronidazole Menggunakan Kombinasi Kitosan Dan Natrium Karboksimethylselulosa. Jurnal Sains Dan Informatika. 2020;2:151–8. https://doi.org/10.22216/jsi.v4.
Samanthula KS, Kumar CB M, Bairi AG, Satla SR. Development, in-vitro and ex-vivo evaluation of muco-adhesive buccal tablets of hydralazine hydrochloride. Braz J Pharm Sci. 2022;58. https://doi.org/10.1590/s2175-97902020000318635.
Samanthula KS, Bairi AG, Mahendra Kumar C. Muco-adhesive buccal tablets of candesartan cilexetil for oral delivery: preparation, in-vitro and ex-vivo evaluation. J Drug Deliv Ther. 2021;11:35–42. https://doi.org/10.22270/jddt.v11i1-s.4547.
Permana AD, Mir M, Utomo E, Donnelly RF. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: a proof of concept study. Int J Pharm X. 2020;2:100047. https://doi.org/10.1016/j.ijpx.2020.100047.
Pereira MN, Reis TA, Matos BN, Cunha-Filho M, Gratieri T, Gelfuso GM. Novel ex vivo protocol using porcine vagina to assess drug permeation from mucoadhesive and colloidal pharmaceutical systems. Colloids Surf B Biointerfaces. 2017;158:222–8. https://doi.org/10.1016/j.colsurfb.2017.07.008.
Mirza MA, Ahmad S, Mallick MN, Manzoor N, Talegaonkar S, Iqbal Z. Development of a novel synergistic thermosensitive gel for vaginal candidiasis: an in vitro, in vivo evaluation. Colloids Surf B Biointerfaces. 2013;103:275–82. https://doi.org/10.1016/j.colsurfb.2012.10.038.
Parikh SK, Patel AD, Dave JB, Patel CN, Sen DJ. Development and validation of UV spectrophotometric method for estimation of itraconazole bulk drug and pharmaceutical formulation. Int J Drug Dev Res. 2011.
Deshmukh AG. Simultaneous estimation of itraconazole and terbinafine HCl in bulk and pharmaceutical tablet dosage form by using UV spectrophotometric method. Int J Pharm Pharm Res. 2019.
Chavan P, Bandgar S, Gejage S, Patil S, Patil S. Development and validation of uv spectrophotometric method for estimation of itraconazole in bulk drug and solid dosage form. Asian J Pharm Res. 2021;11:13–6. https://doi.org/10.5958/2231-5691.2021.00004.6.
Cazorla-Luna R, Martín-Illana A, Notario-Pérez F, Miguel Bedoya L, Tamayo A, Ruiz-Caro R, et al. Vaginal polyelectrolyte layer-by-layer films based on chitosan derivatives and Eudragit® S100 for pH responsive release of tenofovir. Mar Drugs. 2020;18:1–22. https://doi.org/10.3390/md18010044.
Singh G, Pai RS. Trans-resveratrol self-nano-emulsifying drug delivery system (SNEDDS) with enhanced bioavailability potential: optimization, pharmacokinetics and in situ single pass intestinal perfusion (SPIP) studies. Drug Deliv. 2015;22:522–30. https://doi.org/10.3109/10717544.2014.885616.
Drescher S, van Hoogevest P. The phospholipid research center: current research in phospholipids and their use in drug delivery. Pharmaceutics. 2020;12:1–36. https://doi.org/10.3390/pharmaceutics12121235.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:1–10. https://doi.org/10.5402/2012/195727.
Maincent JP, Najvar LK, Kirkpatrick WR, Huang S, Patterson TF, Wiederhold NP, et al. Modified release itraconazole amorphous solid dispersion to treat Aspergillus fumigatus: importance of the animal model selection. Drug Dev Ind Pharm. 2017;43:264–74. https://doi.org/10.1080/03639045.2016.1236811.
Tang J, Bao J, Shi X, Sheng X, Su W. Preparation, optimisation, and in vitro–in vivo evaluation of febuxostat ternary solid dispersion. J Microencapsul. 2018;35:454–66. https://doi.org/10.1080/02652048.2018.1526339.
Soliman KA, Ullah K, Shah A, Jones DS, Singh TRR. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discov Today. 2019;24:1575–86. https://doi.org/10.1016/j.drudis.2019.05.036.
Hecht H, Srebnik S. Structural characterization of sodium alginate and calcium alginate. Biomacromol. 2016;17:2160–7. https://doi.org/10.1021/acs.biomac.6b00378.
Tentor F, Siccardi G, Sacco P, Demarchi D, Marsich E, Almdal K, et al. Long lasting mucoadhesive membrane based on alginate and chitosan for intravaginal drug delivery. J Mater Sci Mater Med. 2020;31. https://doi.org/10.1007/s10856-020-6359-y.
Yanuar RF, Sulaiman TS, Kuncahyo I. Optimization of formulation tablet nifedipine sustained-release combination sodium alginate and HPMC K15M as mucoadhesive polymers with simplex lattice design. Jurnal Farmasi Indonesia. 2015. Vol 12 No 1
Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23:2709–28. https://doi.org/10.1007/s11095-006-9104-4.
Saski W, Shah SG. Availability of drugs in the presence of surface-active agents I.Critical micelle concentrations of some oxyethylene oxypropylene polymers. J Pharm Sci. 1965;4(1). https://doi.org/10.1002/jps.2600540117
European Medicine Agency. ICH Q14 Analytical procedure development - Scientific guideline. Science Medicines Health. 2022.
Thapa R, Gurung S, Parat MO, Parekh HS, Pandey P. Application of sol–gels for treatment of gynaecological conditions—physiological perspectives and emerging concepts in intravaginal drug delivery. Gels. 2022;8. https://doi.org/10.3390/gels8020099.
Andreani T, Miziara L, Lorenzón EN, de Souza ALR, Kiill CP, Fangueiro JF, et al. Effect of mucoadhesive polymers on the in vitro performance of insulin-loaded silica nanoparticles: interactions with mucin and biomembrane models. Eur J Pharm Biopharm. 2015;93:118–26. https://doi.org/10.1016/j.ejpb.2015.03.027.
Willems HME, Ahmed SS, Liu J, Xu Z, Peters BM. Vulvovaginal candidiasis: a current understanding and burning questions. J Fungi. 2020;6. https://doi.org/10.3390/jof6010027.
Squier CA, Mantz MJ, Schlievert PM, Davis CC. Porcine vagina ex vivo as a model for studying permeability and pathogenesis in mucosa. J Pharm Sci. 2008;97:9–21. https://doi.org/10.1002/jps.21077.
Acknowledgements
The authors are grateful to the Indonesia Endowment Funds for Education (LPDP) for the scholarship master’s degree in Pharmaceutical Science at Hasanuddin University. The authors are also grateful to Sesilia R Pakadang and St. Ratnah for the assistance provided in the ex vivo studies. The authors are also thankful to PT Tresna Abadi Sempurna as a LIPOID supplier who has kindly provided Phospholipon® 90G.
Funding
This study was supported by the Ministry of Education, Culture, Research, and Technology of Indonesia through the grant of “Penelitian Tesis Magister” (090/E5/PG.02.00/PT/2022).
Author information
Authors and Affiliations
Contributions
Muli Sukmawaty: conceptualization, methodology, data analysis, original draft writing, editing. Sartini: methodology, writing review, investigation. Andi Dian Permana: conceptualization, methodology, writing review, supervision. Mukarram Mudjahid: editor, writing review. Tri Puspita Roska: editor, writing review. Latifah Rahman: conceptualization, methodology, investigation, writing review, supervision, funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval
We have no ethical issue to declare.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sukmawaty, M., Sartini, Permana, A.D. et al. Lipid Complex-Itraconazole in Thermosensitive Mucoadhesive Vaginal Gel to Enhance the Effectiveness of Therapy for Vulvovaginal Candidiasis: Formulation, Optimization, Characterization, and Ex vivo Evaluation. J Pharm Innov 18, 1546–1559 (2023). https://doi.org/10.1007/s12247-023-09738-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09738-1