Abstract
Purpose
Over the past decade, successive outbreaks and epidemics of infectious diseases have challenged the emergency preparedness and response systems of global public health institutions, a context in which vaccines have become the centerpiece to strengthening global health security. Nevertheless, vaccine research and development (R&D) is a complex, lengthy, risky, uncertain, and expensive process. Alongside strict, time-consuming regulatory compliance, it takes multiple candidates and many years to register a new vaccine. This is certainly not welcome in a global health crisis such as the COVID-19 pandemic. Therefore, this study aims to understand the R&D paradigm shift in pandemic contexts and its impacts on the value chain of vaccine innovation.
Methods
To that end, this paper carried out a systematic literature review and meta-synthesis of 27 articles and reports (2011–2021) that addressed vaccine R&D in contexts of global health threats, disease outbreaks, epidemics, or pandemics.
Results
The research findings are synthesized in a meta-model, which describes a fast-track R&D for pandemic contexts, its driving forces, innovations, mechanisms, and impacts in the value chain of vaccine innovation.
Conclusions
The study demonstrates that, in pandemic contexts, a fast-track R&D process based on close collaboration among regulators, industry, and academia and leveraging enabling technologies can drastically reduce the time required to bring safe, stable, and effective vaccines to market by an average of 11 years compared to the traditional R&D process. Furthermore, pharmacovigilance and rigorous monitoring of real-world evidence became critical to ensuring that quality and safe products were authorized for use during a pandemic.
Similar content being viewed by others
Introduction
Infectious diseases account for one-third of the leading causes of death worldwide [1]. Over the past decade, successive outbreaks and epidemics of infectious diseases, such as H1N1 influenza, Ebola, Zika, and now COVID-19, have challenged the emergency preparedness and response systems of global public health institutions [2,3,4]. In this context, immunization is the most effective public health intervention for preventing infection and/or reducing the severity of morbidity and mortality from infectious diseases [5]. Nonetheless, ensuring global vaccine access is challenging [6,7,8]. Among the numerous limiting factors, vaccine research and development (R&D) stands out as a complex, lengthy, risky, uncertain, and expensive process [9]. Due to strict compliance with regulatory standards required to ensure safety, efficacy, and quality, as well as the high R&D costs and potential for failure, developers typically follow a linear sequence of steps, with multiple pauses for data analysis or manufacturing process checks [3]. Despite the intense debate around streamlining the R&D process since the mid-1990s, the exploration of new technologies, and the implementation of programs by regulatory authorities such as the U.S. Food and Drug Administration (FDA) to expedite the R&D process that began in the mid-twenty century [10, 11], it still takes several years to register a new vaccine [12]. In exceptional conditions, such as in outbreaks or rare diseases, regulatory authorities, not without critical assessments [13], have implemented strategies like compassionate use or expanded access to investigational drugs [14], rolling submission [15], and fast-track or on-prime regulatory pathway approaches [16]. Such strategies require innovative mechanisms and close interaction between manufacturers and regulators.
Other factors, such as the industry’s oligopolistic (often monopolistic) nature, market dynamics, demand conditions, health care infrastructure, macroeconomic instability, non-tariff and trade barriers, and intellectual property, have a profound effect on the international division of labor along the entire biopharmaceutical value chain, thus concentrating vaccine production and technology generation in developed countries [17, 18]. Moreover, low- and middle-income countries are hindered by other developmental issues that restrict their ability to invest in building local capabilities [19, 20]. Such a context raises significant barriers to increasing the global production capacity and results in uneven technological development among countries and inequitable access to vaccines [8]. However, in a worldwide health crisis, where therapeutics and preventive vaccines must be developed, manufactured, and delivered rapidly and efficiently, such a complex environment is certainly not welcome [12].
In this regard, research efforts have been made toward understanding the impacts of R&D in the value chain of vaccine innovation. Most recently, the Pharmaceutical Value Chain Model by Biswas [21] linearly described the R&D stages for pharmaceutical compounds from target to launch. Likewise, the Drug Discovery, Development, and Deployment Maps by Wagner et al. [22] thoroughly represented the therapeutic development processes for biologicals. In turn, the Valorization and Technology Transfer Cycle by Ribeiro et al. [23] provided a circular model that includes market and society domains feeding into science and business development domains. Finally, the Vaccine Innovation Cycle by Van de Burgwal et al. [24] integrated a complex array of steps required for vaccine development into a cross-domain innovation model. Nevertheless, these models have been developed within a non-pandemic or traditional context, making them limited in describing the technological and process innovations required to develop, manufacture, and deliver vaccines rapidly.
Other R&D models have also been proposed considering contexts of global health threats, disease outbreaks, epidemics, or pandemics. As proof, the seminal works by Michael Kremer [25, 26] offered demand-pull mechanisms to leverage vaccine R&D. On the technology push, the Call Options for Vaccines Model by Brogand and Mossialos [27] stimulated research into neglected diseases based on the concept of a financial call option. The FastVax Design Model by De Groot et al. [28] suggested using computational tools to design and deliver vaccines on demand for biodefense purposes. The Papaneri et al. [29] model provided a pathway for expediting vaccine development against emerging diseases. The Vaccine Ecosystem proposed by Saadatian-Elahi et al. [30] described the main actors involved in vaccine R&D, manufacturing, distribution, procurement, and immunization. In turn, the Process Mapping Vaccines by Drury, Jolliffe, and Mukhopadhyay [31] captured the key, rate-limiting bottlenecks in vaccine R&D and recommended parallel steps for cases of epidemic threats. Lastly, the model by Lurie et al. [3] proposed a fast-track R&D with parallel steps for developing vaccines at pandemic speed. The problem is that these models are primarily deductive in nature, and empirical evidence supporting their adoption is missing. Furthermore, they do not present a typology of innovations and mechanisms depicting the differences from traditional models or the effects of those differences. Finally, compared to traditional models, besides offering a broader level of granularity, the outbreak/epidemic/pandemic models do not contemplate the entire extent of the value chain of vaccine innovation.
Given the above, this work poses the following research questions: (i) Which models of vaccine R&D consider pandemic contexts in their design? (ii) What are the differences between vaccine R&D in traditional and pandemic contexts? (iii) Which forces drove the change of vaccine R&D from traditional to pandemic contexts? (iv) Which innovations made it possible to shift vaccine R&D from traditional to pandemic contexts? (v) Which mechanisms might explain the changes brought about by these innovations? (vi) How might the changes in R&D from traditional to pandemic contexts affect the value chain of vaccine innovation?
This paper aims to answer these questions through a systematic literature review and meta-synthesis of 27 articles and reports (2011–2021) encompassing research in medicine, immunology, microbiology, pharmacology, toxicology, and pharmaceutics. The research findings are synthesized in a vaccine innovation meta-model composed of (i) a fast-track vaccine R&D model; (ii) an innovation causal model; and (iii) a prospective framework. The first model inductively describes a fast-track vaccine R&D for pandemic contexts. This model comprises ten workstreams, 29 stages, 27 gates, and ten innovations. The second model, in turn, connects six enablers, nine driving forces, ten innovations, 15 mechanisms, and 15 outcomes to explain the shift of vaccine R&D from traditional to pandemic contexts. Finally, the prospective framework groups 20 guidelines supporting future vaccine innovation models.
The remainder of this paper is structured as follows. The “Systematic Literature Review” section outlines the methodological research procedures. The “Reference Models” section describes the reference models underpinning this work. The “Vaccine Innovation Meta-Model for Pandemic Contexts” section synthesizes the systematic literature review results through a vaccine innovation meta-model for pandemic contexts. The “Discussion of the Results” section critically analyzes the research findings, followed by the “Closing Remarks” section, which closes the articles by providing the research contributions, limitations, and future opportunities.
Systematic Literature Review
This work is configurative and seeks to produce analytically generalizable scientific knowledge through the synthesis of existing research. For this reason, it follows the five-stage systematic literature review by Ermel et al. [32], as outlined in Fig. 1.
The process started by defining vaccine R&D for pandemic contexts as the core research subject. Then, in step 1.1 of Fig. 1, the protocol for systematic literature review was developed according to Table 3 in Appendix, as reasoned by Ermel et al. [32]. Subsequently, in step 1.2 of Fig. 1, the protocol was assessed for bias by two experts in vaccine R&D from Bio-Manguinhos/Fiocruz Immunobiological Technology Institute, who were not part of the team that conducted this work. The bias assessment followed the rationale of the ROBIS technique proposed by Whiting et al. [33] and later adapted by Ermel et al. [32] for use in systematic literature review protocols. The assessment consists of answering ten questions related to the scope, theoretical framework, and eligibility criteria, among other aspects of the review, which can result in a “low,” “high,” or “unclear” bias score. Any result other than low triggers the protocol to a new review/assessment cycle. After refinements regarding the research questions, the protocol scored a low risk of bias and was used in the review stage.
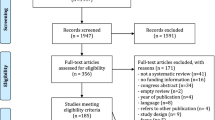
This work performed a three-step search and eligibility process, which includes (2.1) database searching, (2.2) snowballing, and (2.3) experts’ contacting. Concerning the databases (step 2.1 in Fig. 1), the search was conducted in Scopus, Web of Science, and Science Direct, wherein only peer-reviewed articles published up to 2021 were consulted. To identify those studies related to innovation models and health emergency/pandemic contexts, the search was limited to article titles, abstracts, and keywords, resulting in 674 studies across the three databases. Duplicates (166) were discarded, followed by an inspection of titles and abstracts, as recommended elsewhere [34]. Then, 139 potentially relevant studies were analyzed in-depth, and 21 within the research scope and quality requirements were considered in the snowballing search (step 2.2 in Fig. 1). The snowballing was conducted backward and forward, as reasoned by Wohlin [35]. From a reference and a citing list composed of 894 and 911 records, respectively, 1.805 studies were identified. After removing the duplicates (363), a total of 1.442 studies were inspected, of which 12 studies were read in-depth, and five following the eligibility and quality criteria were considered for inclusion. In step 2.3 of Fig. 1, 26 articles comprising the preliminary corpus of analysis were assessed by the experts mentioned above, which recommended the inclusion of 1 reference not found in previous review steps. As a result, 27 studies (R) were selected to compose the corpus of analysis, as presented in Table 1. It is noteworthy that the time frame (2011–2021) was not a search criterion, as shown in the protocol (Appendix, Table 3), but rather resulted from the eligibility process. Nevertheless, during this process, special attention was placed on works published after the occurrence of other outbreaks, epidemics, or pandemics (e.g., SARS, swine flu, and MERS). The works retrieved before 2011, however, did not intend to propose an innovation model, but rather discuss specific critical success factors along the R&D value chain and/or public policies. Further details on data reduction are provided in Appendix (Fig. 6, Tables 4 and 5).
Step 3.1 of Fig. 1 consisted of encoding the corpus of analysis, which was undertaken by iteratively defining the codes, aggregating the codes into categories, and then assigning the codes and categories to the full texts [32]. This work adopted a mixed encoding scheme composed of categorical and open codes, as well as a priori and a posteriori categories [51]. The categorical codes and a priori categories were defined before the full-texts reading and were based on Vaccine Innovation Cycle (VIC) by Van de Burgwal et al. [24] and CIMO-logic by Denyer, Tranfield, and Van Aken [52]. Both models and the justification for their choice are summarized in the “Reference Models” section. The open codes and a posteriori categories, in turn, emerged during the in-depth analysis, as reasoned by Strauss and Corbin [53]. Concerning the rationale for grouping codes into categories, this work adopted the thematic criterion and followed the principles of mutual exclusion, homogeneity, pertinence, objectivity, and productivity, as posed by Bardin [54]. As a result, ten workstreams (W), 29 stages (S), 27 gates (D, U, and T), two critical incidents (C), six enablers (E), nine driving forces (F), ten innovations (I), 15 mechanisms (M), 15 outcomes (O), and 20 guidelines (G) emerged from the encoding, as presented in Tables 2 and 6 (Appendix).
Next, considering that the more frequently cited, the more important the code/category is [55], a thematic analysis was performed in step 3.2 of Fig. 1. In this step, the absolute frequencies of W, S, D, U, T, C, E, F, I, M, O, and G were accounted for and then disposed of in an occurrence matrix, as shown in Appendix (Table 7). In parallel, a structural analysis was performed in step 3.2 of Fig. 1, according to Bardin [54]. First, the workstreams, stages, and gates resulting from the encoding process were organized according to the Vaccine Innovation Cycle by Van de Burgwal et al. [24], but linearly. Considering the traditional R&D process, the duration of the stages was established based on the works by Biswas [21], Velho et al. [56], Van de Burgwal et al. [24], and Plotkin et al. [9]. In light of the emergency/pandemic contexts, the stages had their duration and temporal disposition adjusted based on the studies composing the corpus of analysis, as well as the timelines of the COVID-19 pandemic from the American Journal of Managed Care (AJMC) and World Health Organization (WHO) [57, 58]. Finally, the innovations identified in step 3.1, primarily related to, but not limited to, the COVID-19 pandemic, were linked to their respective stages, giving rise to the first partial result of this research, the fast-track vaccine R&D model, as outlined in Fig. 2. Aiming to explain the shift from the traditional R&D to the one proposed for pandemic contexts, a cause-and-effect analysis was performed among the enablers, driving forces, innovations, mechanisms, and outcomes retrieved from step 3.1, as reasoned by Denyer, Tranfield, and Van Aken [52], and portrayed in Fig. 3. In this sense, through the occurrence matrix coming from step 3.2 (Table 7), a co-occurrence matrix was elaborated (Table 8) based on the simultaneous occurrence (co-occurrence) of two codes/categories in the same study [54]. Since the co-occurrence does not always imply causation [59], each pairwise relationship identified from Table 8 was checked for consistency by following the principles of causality existence, causality clarity, the sufficiency of cause, and additional cause retrieved from the Theory of Constraints Thinking Process [60, 61]. The co-occurrence relationships that did not show causation—those appearing in Table 8 but not in Table 9—were discarded, giving rise to a causation matrix (Table 9), from which the second partial result of this study was elaborated, the innovation causal model presented in Fig. 3. Finally, the guidelines and the potentially impacted stages were abductively linked to build the prospective framework for future innovation models, considered in this work as the third partial result.
Finally, step 4.1 of Fig. 1 consisted of a meta-synthesis based on the final stages of the works by Walsh and Downe [62] and Noblit and Hare [63]. Here, (i) the fast-track vaccine R&D model, (ii) the innovation causal model, and (iii) the prospective framework resulting from the structural analysis (step 3.3 in Fig. 1) were connected into a vaccine innovation meta-model for pandemic contexts, as presented in the “Vaccine Innovation Meta-Model for Pandemic Contexts” section. This process was undertaken by a continuous comparative assessment of studies until a comprehensive understanding of the meta-model was realized. Right after, in step 4.2 of Fig. 1, the sufficiency of the meta-model was assessed by the same experts in vaccine R&D until it reached saturation, as reasoned by Eisenhardt [64]. Finally, the results presented in the “Vaccine Innovation Meta-Model for Pandemic Contexts” section close stage 5 of this research. The limitation of the research design adopted here lies in the fact that some work might have been overlooked, which does not invalidate the contribution of this article toward the existing body of knowledge of vaccine innovation models.
Reference Models
Two reference models were used as a conceptual basis to answer the six research questions posed in the “Introduction” section. The first was the Vaccine Innovation Model (VIC) by Van de Burgwal et al. [24], which combines the principle of stage-gates by Cooper [65] with the Valorization and Technology Transfer Cycle by Ribeiro et al. [23] to provide a cross-domain understanding on the value chain of vaccine innovation. The VIC comprises 29 stages and 28 gates distributed across ten interconnected workstreams, depicted circularly, as further detailed by Figures 7, 8, 9 and 10 (Appendix). The stage-gates are classified as defined (D), undefined (U), and monitoring (M). The defined stage-gates take place in a predictable order and timing. The undefined stage-gates have their occurrence and timing determined by various factors, whereas the monitoring stage-gates occur continuously and iteratively. This model was chosen for its ability to holistically represent, beyond the firm’s boundaries, the value chain of vaccine innovation at an appropriate level of granularity. The second model used was the CIMO-logic by Denyer, Tranfield, and Van Aken [52], which combines the logic of prescription and causality to explain the mechanisms by which intervention in a given context gives rise to specific outcomes. The model is composed of four components: (i) context (C), which represents the surrounding factors influencing the change; (ii) interventions (I), which relate to the elements intended to trigger the change; (iii) mechanisms (M), which refers to the mechanics of change; and (iv) outcomes (O), which account for the results stem from the change. Further details on CIMO are provided in Table 10 (Appendix). This model was chosen because of its ability to establish causal relationships qualitatively and its flexibility of instantiation to different contexts. The following section presents the vaccine innovation meta-model for pandemic contexts.
Vaccine Innovation Meta-Model for Pandemic Contexts
This section presents the vaccine innovation meta-model for pandemic contexts, which is composed of three entities: (i) the fast-track vaccine R&D model; (ii) the innovation causal model; and (iii) the prospective framework. The fast-track vaccine R&D model depicts the reorganization of the activities required to develop, manufacture, and deliver safe, stable, and effective vaccines at pandemic speed. The model was built upon the Vaccine Innovation Cycle by Van de Burgwal et al. [24] and is composed of ten workstreams, 29 stages, 27 gates, and ten innovations, as shown in Fig. 2 and detailed in Table 6 (Appendix). Although the original proposition by Van de Burgwal et al. [24] is presented circularly, in this work, the model is depicted linearly to highlight the sequencing and duration of the stage-gates. In Fig. 2, the horizontal lanes represent the workstreams (W), which group stages of the same nature and purpose, while the vertical lanes outline the time intervals in years. The stages (S) consist of the best-practice activities needed to progress the project to the next decision point and are represented in Fig. 2 by the horizontal bars. The highlighted bars show the sequencing and duration of the stages in the pandemic context, which were defined based on the interpretation of the arguments present in the studies comprising the corpus of analysis, as well as on the timelines of the COVID-19 pandemic from the AJMC and WHO [57, 58]. The faded bars, in turn, show the sequencing and duration of the stages in the traditional context, which were established from the works by Biswas [21], Velho et al. [56], Van de Burgwal et al. [24], and Plotkin et al. [9]. The circles represent the points where the path forward for the next stage is agreed to, named gates (D, U, and TFootnote 1), and the values between parentheses indicate the absolute frequency of the stage-gates within the corpus of analysis. The time lags between the highlighted and faded stage-gates show the differences between the traditional and pandemic vaccine R&D paradigms. Finally, the hexagons exhibit the innovations (I) triggering the differences between the R&D paradigms, and the diamonds (C) represent critical incidents, which consist of events associated with the pandemic context.
The second entity, the innovation causal model, explains how innovations, driven by the pandemic and supported by contextual factors, enabled the R&D paradigm shift and made it possible to develop, manufacture, and deliver vaccines rapidly. Based on the CIMO-logic by Denyer, Tranfield, and Van Aken [52], the model is organized into four layers, as shown in Fig. 3. The first layer represents the context and is subdivided into two elements, the driving forces (F) and enablers (E). The driving forces refer to the motivators of the shift from the traditional to the pandemic R&D paradigm, while the enablers consist of the contextual factors that made the paradigm shift possible. The second layer refers to interventions, which in this research assume the form of innovations (I) that trigger the change. They configure central elements in the meta-model since they establish the bridge between the R&D fast-track model (Fig. 2) and the innovation causal model (Fig. 3). The third layer relates to the mechanisms (M), i.e., the mechanics underlying the change. Said otherwise, the mechanisms explain how the innovations transformed the traditional R&D paradigm into the pandemic one and generated their respective outcomes. Finally, the last layer refers to the outcomes (O). The outcomes point out the effects, whether positive or negative, coming from the R&D paradigm shift. The enablers (E), driving forces (F), innovations (I), and outcomes (O) are represented in Fig. 3 by rectangles, each one with its corresponding color and absolute frequency. These elements are defined in Appendix (Table 6). Lastly, the connections between the elements (e.g., E → I, F → I, I → M, and M → O) are represented by a continuous arrow, called relationships.
The last entity of the meta-model is the prospective framework (Table 2), consisting of a set of actions capable of stimulating future innovations, which are referred to as guidelines (G). The guidelines were classified into five domains [4]: (i) technological, i.e., techno-scientific knowledge, application, tools, and techniques; (iv) policy, i.e., governmental or supra-governmental rules, laws, directives, and programs; (iii) process, i.e., activities and tasks; (iv) infrastructure, i.e., physical resources, consumables, services, and human capabilities; and (v) institutional, i.e., norms, values, and behavior.
At the technological level, the main challenges include the existing R&D and innovation infrastructure, access to consumables, equipment, and services, high fixed costs, and investment in GMP manufacturing facilities, as well as financing instruments. Depending on the pathogen, the technology platform, and other factors, various issues may arise, primarily related to biosafety measures. Moreover, economic scale and scope, as well as demand conditions, become important drivers for investments in new technologies and facilities. In poor resource settings, these challenges become more pronounced since they still face developmental issues already overcome by rich countries. The COVID-19 pandemic exemplified these issues, prompting intense debates about intellectual property, global value chains, technology, and economic dependency. At the policy level, countries are challenged to allocate resources and redirect research priorities, while maintaining other state investments, which traditionally form the basis of a robust and innovative health research and innovation system. To approach prosperity and development differently, resource allocation and investment decisions should be based on the well-being of society, rather than other interests. Intellectual property is a significant barrier to entry, and should be addressed at the policy level. Although a patent waiver at the onset of the outbreak might allow some emerging countries with research and manufacturing capabilities to access new technologies, this is not a permanent and viable solution. At the process level, challenges are associated with establishing agile and clear processes at a system level so that the necessary policy instruments and resources can be implemented at the beginning, and health decisions are based on quality and reliable data. Regarding infrastructure, the main challenge is sustaining a steady flow of investments in R&D and manufacturing capacity building since preparedness necessitates long-term commitments. Lastly, the COVID-19 pandemic highlighted the importance of considering social and cultural factors which influence the decision-making regarding vaccine uptake, and the support of immunization programs at the institutional level.
In addition to domain classification, the guidelines were also linked to the stages they could potentially affect. This association bridges the prospective framework (Table 2) to the fast-track vaccine R&D model (Fig. 2), thus completing the meta-model, as illustrated in Fig. 4. The rationale underlying the connection of the three models is described, by workstreams, in the following subsections.
Research and Development (W1)
This workstream combines nine vaccine R&D-related stage-gates, which starts with the unmet needs assessment (S1-D1) and scope preparation (S2-D2), moves through the exploration and discovery (S3-D3), progresses to preclinical development (S4-D4 and S5-D5), and ends with the application and execution of phase I, phase II, and phase III clinical trials (S6-D6:S9-D9) [24]. In the traditional R&D paradigm, these stage-gates are executed sequentially, lasting approximately ten years [9], as indicated by the faded bars in Fig. 2. In the pandemic R&D paradigm (highlighted bars in Fig. 2), this duration could be reduced to approximately one year due to three innovations, as seen in the COVID-19 pandemic: (I7) nucleic acid (DNA/RNA) platform; (I8) recombinant viral vector platform; and (I6) adaptive clinical trial design.
Advances in biotechnology and molecular biology (E3) [4], combined with ongoing research for nearly a decade in preparation for infectious diseases of pandemic potential (E2) [3], allowed the design of new technological platforms such as nucleic acid (DNA/RNA) (I7) and recombinant viral vector (I8) [47]. The need to rapidly develop safe and effective vaccines to halt pandemic advances (F3) required new R&D strategies (F7) [49], which, in the case of the COVID-19 pandemic, resulted in the possibility of using vaccine platforms not having the established track-record of classical approaches [17]. The use of genetic modifications to combat pathogenic mechanisms enabled the rapid adaptation (M10) of these platforms to new viruses, thus reducing in almost two years the duration of the exploration and discovery phase (O7) of new candidate vaccines for COVID-19 [42]. Another benefit of using these platforms was the existence, in some cases, of already demonstrated safety profiles [17], thus making it possible to shortcut the preclinical development and move directly into clinical development, reducing even more (≈ 2 years) the R&D lead time (O11). The precedent of using new platforms, coupled with their high adaptability (M10), also made it possible for new companies to enter the vaccine segment (M11) [46], increasing the number of candidate vaccines (M12) and, therefore, the chance of having safe and effective vaccines (O8) at the end of the R&D process [4].
Lower R&D lead times were not only related to technological innovations (I7 and I8), but also to process innovations, such as the adaptive and decentralized clinical trial design (I6) [45]. Enabled by flexible regulatory pathways (F4) [41], conditioned by high levels of community transmission (F2) [2], and supported by the possibility of changing trial protocol parameters according to observations, adaptive clinical trials allowed parallelism among clinical trial phases (M6) and, in some cases, enabled the merge between them (e.g., Phases I/II and II/III) [44]. Executing randomized control trials (RCT) in parallel reduced the duration of clinical development (O6) by approximately five years, accounting for the largest share of total R&D lead time. However, lower R&D lead times did not come at zero cost. If, on the one hand, in the case of DNA/RNA platforms, not using cultures or fermentation reduced the manufacturing complexity (M8) and facilitated their reproducibility and validation (M9) to the point of lowering costs (O4) and GMP lead time (O5) [3]. On the other hand, it required the use of ultra-cold chain technology (M7) [50], which increased not only manufacturing but also logistics costs (O4). Moreover, the limited number of companies with technological mastery of the new platforms further underscored the need for expansion and modernization of manufacturing systems worldwide (M13) [5], which by the end of 2021 had not yet reached the capacity to meet the global demand arising from the pandemic of COVID-19 (O9). Finally, although new vaccines have had adverse effects, such events were considered rare and not above what is observed in other cases.
The paradigm shift enabled a reduction of approximately nine years in vaccine R&D, but there are several opportunities for improvements, as pointed out by some guidelines of the prospective framework (Table 2). In technological terms, conducting countermeasure research to improve knowledge and develop databases of potential targets and simulation algorithms (G1) may facilitate the exploration and discovery (S3-D3) of future vaccine candidates [17]. Likewise, in cases where there are no safety profiles already demonstrated, the adoption of in-silico clinical trials based on digital technologies (G3) might reduce, refine, or partially replace preclinical and clinical trials (S4-D4:S9-D9) [48]. From a process perspective, standardizing clinical trial procedures (G11) might enable the test of multiple vaccine candidates against a single control arm [18], facilitate data sharing and comparison of results across studies [50], and eliminate redundant activities [45], among other benefits. Finally, the technological and process guidelines are accompanied by other infrastructure guidelines, such as (i) the establishment of a global genetic sequence repository (G13) to share the genetic sequencing of potential pandemic pathogens [2]; (ii) the sharing of continent-based virus sharing networks (G14) to receive, verify, store, and share virus isolates locally [44]; (iii) the building of a containment laboratories network (G15) to validate animal models [44]; (iv) the development of continent-based clinical and biological sample networks (G16) to locally collect, store, and share clinical and biological samples [43]; and (v) the establishment of country-based clinical supercenters (G17) to recruit patients, manage trials, and collate trial data [45].
Good Manufacturing Practices (GMP) (W2)
Composed of two stage-gates, chemistry, manufacturing, and control (CMC) (S10-D10) and prepare manufacturing (S11-D11), this workstream comprises the activities and decisions concerning the manufacturing preparation to meet good manufacturing practices (GMP) [24]. In the traditional R&D paradigm, these stage-gates are performed in series, usually precede clinical trials (S7-D7:S9-D9), and have an aggregated duration of about four years (faded bars in Fig. 2) [9]. In the pandemic R&D paradigm, their execution remained serial, but progressed in parallel with clinical trials, even with a reduction of approximately three years in lead time, as seen in Fig. 2.
Two innovations might explain the short duration of these activities: (i) partnership building (I9) and (ii) extraordinary GMP certification (I4), such as those under the International Council for Harmonization (ICH), like the Pharmaceutical Inspection Co-operation Scheme (PIC/S). Stimulated by the need to develop alternative strategies for upscaling production (F8), the partnerships were built through technology transfers [12], mainly between developers and contract manufacturing organizations (CMO) and, in some cases, between developers and state-owned manufacturers, such in Influenza H1N1 outbreak [37]. The partnership building, besides helping to expand local (M13) and global production capacity (O9) [49], also made it possible to reduce manufacturing costs (O4) by sharing the risks (M14) inherent in the early stages of R&D (S4-D4:S8-D8) [47]. In turn, the extraordinary GMP certification (I4), empowered by flexible regulatory pathways (F4) and supported by legal reference mechanisms (E4), enabled, through remote inspections and the use of certificates issued by other regulators (M5), reductions in GMP (O5) and licensing lead times (O2) with the drawback of increasing ethical and regulatory risks [4].
Unfortunately, concerning the COVID-19 vaccines, the production capacity did not and still does not meet the global demand, particularly in low- and middle-income countries. From a process standpoint, one way to reverse such undesirable effects is through strengthening technology transfer (G8), either by direct transfer, via hubs, or through capacity building [36, 47], as pointed out in the prospective framework (Table 2). Another alternative, but of an infrastructure nature, that may help increase domestic production, reduce costs, and accelerate the time-to-market of new vaccines is the establishment of global factory networks (G18) [5].
Market, Preparation, Registration, and Introduction (W3)
This workstream comprises the stage-gates related to the introduction of vaccines on the market, which begins with market preparation (S12-D12), goes through registration (S13-D13), advances to national implementation (S14-D14), and ends with market deployment (S15-D15) [24]. These stage-gates occur serially in the traditional R&D paradigm, with the beginning of registration (S13-D13) conditional on completing the RCT Phase III (S9-D9). In the pandemic R&D paradigm, the stage gates of the workstream W3 were executed in parallel to each other and to the clinical development-related stage-gates (S6-D6:S9-D9). In the case of the COVID-19 pandemic, the parallelism of stage-gates from S4-D4 to S14-D14 shortened the vaccine time-to-market (O12) by approximately 11 years, being the main outcome of the R&D paradigm shift. In this workstream, five innovations contributing to the reduction in time-to-market were found: (i) advanced market commitment and direct grants (I1); (ii) extraordinary registration and post-registration petitions (I2); (iii) emergency use authorization (I3); (iv) extraordinary GMP certification (I4); and (v) rolling submission/review (I5).
The financial risks (O10) arising from unfolding new R&D strategies (F7) and flexible regulatory pathways (F4) [42], as well as the historically inequitable vaccine distribution (O1) [47], required alternative financing mechanisms (F5) [5]. Driven by global coordination (E1), these mechanisms (F5) stimulated the cooperation between public and private institutions (F6) [17], which, supported by legal channels (E4), resulted in the adoption of advanced market commitments and direct grants (I1) [18]. In the COVID-19 pandemic, this innovation (I1) ensured not only sustainable demands for the development, manufacture, and delivery of new vaccines (M2) [49], but also secured the partial allocation of doses for low- and middle-income countries as soon as vaccines prove to be effective (M1) [47].
In regulatory terms, the rolling submission/review (I5), combined with the adaptive clinical trials (I6), was instrumental in speeding up licensing times in the COVID-19 vaccines [41]. First, it enabled conducting the phases of clinical development in parallel [49]. Second, it allowed the evaluation of the partial results of some phases in parallel with the execution of others, making it possible to grant emergency use authorization (I3) at the time of its creation for some candidate vaccines [4]. Although this grant made possible the use of vaccines under emergency listing (M4) [7], it was the prior enrollment of these vaccines in the national immunization systems (S14-D14) which permitted their immediate administration in the population (O14) to timely reduce the disease burden (O15). Two other regulatory innovations were also important. The extraordinary GMP certification (I4), as already mentioned above, enabled the adoption of remote inspections and the use of existing certificates, thus reducing the GMP lead time (O5) [28], and the extraordinary registration and post-registration petitions (I2), which enabled the licensing of vaccines that demonstrated safety and efficacy profiles in compliance with the target product profile thresholds set by the WHO, as well as the immediate implementation of active pharmaceutical ingredient (API) related changes (M3) [4].
Although this workstream (W3), together with R&D (W1), has been responsible for a significant reduction in time-to-market (O12) (≈ 7 years), the procedures used by regulatory agencies of different countries remain uncoordinated and inharmonious [18]. This context signals that further progress can be made, such as defining agile regulatory structures and standardized procedures (G10) to enable regulators to exchange symmetric information and make decisions based on the same data [44]. Furthermore, in institutional terms, it is also required the development of tailored and scalable solutions to support national immunization programs (G19) [6, 38].
Funding and Business Development (W4)
This workstream is composed of six stage-gates sharing the goal of funding and business development [24]: (i) funding (S16-U16); (ii) scouting (S17-U17); (iii) intellectual property protection (S18-U18); (iv) spin-off company (S19-U19); (v) partnering (S20-U20); and (vi) acquisition (S21-U21). In contrast to the previous workstreams (W1:W3), even in the traditional R&D paradigm, the stages comprising W4 do not necessarily occur sequentially. Moreover, fulfilling all stage-gates and their execution order is not a requirement for each candidate vaccine, a pattern that was also incorporated into the pandemic R&D paradigm [24]. Two innovations rooted in workstream W4 and influencing stage-gates of other workstreams (W2, W3, and W5) were identified in this context. One was the adoption of advanced market commitments and direct grants (I1) [18], which ensured sustainable demands for vaccine R&D (M2) [49], as well as secured a share of doses for low- and middle-income countries (M1) [47]. The other was the partnership building (I9), which accelerated the expansion and modernization of manufacturing technology and capacity (M13) [49], enabled sharing of the risks inherent in R&D (M14) [47], and allowed, in conjunction with the introduction of new vaccine platforms (I7 and I8), the entry of new companies in the vaccine segment (M11) [46].
However, there is still room for improvement or expansion of some initiatives undertaken in other outbreaks and the COVID-19 pandemic. Without a doubt, the development of mechanisms for a temporary waiver of patents on platform technologies at the outset of the outbreak (G7) would have been welcomed by developing countries with research and manufacturing capabilities, since it could have reduced the cost of vaccines, increased global production capacity [18], and even created a more balanced negotiating landscape for countries participating in demand and pull mechanisms such as the ACT/COVAX and/or pre-procurement or technology transfer agreements concerning intellectual property (IP) protection. Similarly, among other funding-related initiatives, restructuring the financing system (G4) to ensure that the vaccines required for pandemic preparedness, prevention, and response are timely developed, ready, and responsive in the event of the next pandemic would have been more than relevant if in place when COVID-19 emerged [44].
Manufacturing (W5)
This workstream consists of one stage-gate related to manufacturing (S22-U22). Manufacturing vaccines include effective up/downstream processing, quality assurance and control, and the compilation of batch dossiers, which, contingent on GMP conditions, are approved for clinical use [24]. The beginning of the manufacturing stage-gate (S22-U22), conditioned to the end of the preparation stage-gate (S11-D11), remained unchanged in the pandemic R&D paradigm. Compared to the traditional R&D paradigm, the difference is that its duration was shortened to meet the first demands stemming from the emergency use authorization of some candidate vaccines (I3). As well as in the manufacturing preparation (S11-D11), what made it possible to shorten the duration of S22-U22 was the building of partnerships (I9) with CMOs and state-owned producers [49].
In the COVID-19 pandemic, manufacturing systems were able to quickly produce after receiving emergency use authorization, yet global production capacity remains a problem. From an infrastructure perspective, this issue could be addressed by setting up regional and/or global networks of manufacturing facilities (G18) [5], which could be used to meet medical requirements in various contexts during health emergencies. To ensure sustainability in the long term, flexible and multipurpose facilities with high modularity should be prioritized for investment. From a technological perspective, this problem might be addressed with adjuvant technologies (G2). This is because by strengthening the immune response, adjuvants can reduce the frequency of vaccination, making it feasible, with the same global production capacity, to vaccinate more individuals [1].
Market Monitoring (W6)
Composed of two stage-gates, global unmet needs assessment (S23-T23) and demand articulation (S24-T24), this workstream comprises the activities and decisions concerning monitoring, assessment, and prioritization of unmet global needs [24]. In the traditional R&D paradigm, as well as in the pandemic one, the stage-gates within workstream W6 take place continuously and iteratively rather than being preceded by the completion of a previous stage-gate. Furthermore, gates are less formal and sometimes represent outcomes of certain activities rather than fixed criteria against which deliverables of activities are evaluated [24].
In the pandemic R&D paradigm, two critical incidents related to the workstream W6 trigger mobilizations in the vaccine value chain for pandemic preparedness and response. The first is the emergence of an outbreak of an as-yet-unknown infectious disease (C1), which, depending on the rate of community transmission (F2) and impact on health, social, and economic systems (F1), quickly evolves into a pandemic (T23) [4]. In the case of the COVID-19 pandemic, the time lag between C1 and T23 was approximately three months, with the scientific community beginning research activities days after the outbreak was identified. This initiative allowed some candidate vaccines to begin clinical trials concurrently with the pandemic declaration. The second critical incident refers to the emergence of new virus variants (C2), which demands iterations in the R&D process triggered by the prioritization of unmet needs, as illustrated by the feedback between T24 and S2 in Fig. 2 [48]. This feedback reinforces the cyclical nature of innovation models advocated by Van de Burgwal et al. [24].
In this context, besides the initiatives already established or underway, the prospective framework (Table 2) points out some alternatives to make preparedness and response to new pandemics more robust. The first one (G5) is the identification of key areas of research as soon as possible (e.g., G1, G2, G3), and mechanisms in place to quickly release funds from a pre-positioned pool (e.g., G4) to jumpstart the R&D response [44]. The second (G6) consists of the development of an end-to-end preparedness and response ecosystem capable of operating from clinical observation and care, through basic and translational research, to an adequate supply of necessary products widely delivered across the world to diagnose, treat, control, and prevent the disease and bring the outbreak, whatever the cause, to an end [44]. Finally, the last guideline (G9) refers to establishing a framework and threshold for activating R&D preparedness and response for new outbreaks [39].
Innovation Project Monitoring (W7), Portfolio Monitoring (W8), and Product Monitoring (W10)
Three other monitoring workstreams compose the fast-track vaccine R&D model in Fig. 2 [24]: (i) innovation project monitoring (W7); (ii) portfolio monitoring (W8); and (iv) product monitoring (W10). As in W6, these workstreams and their respective stage-gates took place continuously and iteratively in both traditional and pandemic R&D paradigms. Although the workstreams W7 and W8 were not explicitly addressed in the studies reviewed in this research, some related challenges are evident. In the stage-gate of portfolio monitoring (S27), the challenge is to quickly include a new vaccine in the portfolio without impacting the contracted demand for other vaccines from national immunization programs. In the stage-gate of product monitoring (S29), in turn, although there are national actions to enable real-time data collection, from a global standpoint, this process still lacks integrated tools and more uniform protocols (G12) [18], as indicated in the prospective framework (Table 2).
Public Affairs Monitoring (W9)
Composed of one stage, the public affairs (S28) workstream refers to tracking and analyzing media coverage and public opinion on the vaccine. This includes identifying key issues and themes, as well as measuring the level of support, opposition, or hesitancy to vaccination [24]. In this context, anti-vax groups and vaccine hesitancy (F9) have significantly impacted vaccine coverage (O14). The WHO has named lower vaccine uptake as one of the biggest public health problems of our time. To overcome this issue, manufacturers, academia, and multilateral organizations have joined efforts to strengthen awareness and disseminate scientific information about vaccines and immunization through massive digital communication campaigns (E5 and E6). Conveyed through webinars, fast-checking sites, and apps (M15), it helped improve public trust (O13), thus resulting in higher vaccination rates (O14) and, therefore, in reducing the disease burden (O15), which was considered an innovation in COVID-19 pandemic (I10) [12]. Despite the work that has been done, it is still necessary to advance in creating programs and funding, as well as strategies to fight misinformation, bad information, fake news, and communication strategies (G20) [38].
Discussion of the Results
This work presents a vaccine innovation meta-model for pandemic contexts, which was built from a systematic literature review and meta-synthesis of 27 articles and reports (2011–2021) encompassing research on vaccine R&D in situations of global health threats, disease outbreaks, epidemics, or pandemics. The meta-model is composed of three entities: (i) the fast-track vaccine R&D model; (ii) the innovation causal model; and (iii) the prospective framework. The fast-track vaccine R&D model depicts, from the firm to the global level, the reorganization of the activities required to develop, manufacture, and deliver safe and effective vaccines at pandemic speed. Unlike traditional R&D models that bring the defined stage-gates (S1-D1:S15) occurring sequentially [e.g., 21, 22, 24], the first meta-model entity presents most of them running in parallel, as advocated by Lurie et al. [3] and Drury, Jolliffe, and Mukhopadhyay [31]. The beginning of one stage not conditional on the completion of the other, combined with a shorter duration of stage-gates, resulted in a reduction of vaccine time-to-market of approximately 11 years, when compared to the works of Biswas [21], Velho et al. [56], Van de Burgwal et al. [24], and Plotkin et al. [9].
The first entity of the meta-model has its roots in R&D; therefore, it is plausible that the stage-gates belonging to the R&D workstream (W1) appear more frequently in the corpus of analysis. However, in this group, the stage-gate S1-D1 appeared with null frequency (Table 7), an issue that might have overlooked some innovations related to the prioritization of R&D opportunities. Similarly, other stage-gates of null frequency were also identified in the workstreams of innovation project monitoring (W7) and (ii) portfolio monitoring (W8). In W7 and W8, the fact that the related stage-gates appear with zero frequency might have left out of the meta-model some firm-level innovations and mechanisms that enabled both the technology transfer of products still under development, as well as the minimization of impacts on the supply of other vaccines already contracted by national immunization programs. Finally, although the fast-track vaccine R&D model was depicted in a linear fashion, in a pandemic context, the emergence of new variants, represented by the relationship T24 → D2 in Fig. 2, reinforces the cyclical nature of the meta-model as initially posed by Ribeiro et al. [23] and later adopted by Van de Burgwal et al. [24].
The main differences between the meta-model presented in this work and the models developed in contexts of global health threats, disease outbreaks, epidemics, or pandemics [e.g., 3, 28, 29] are the longer extension of the value chain, as well as the identification of innovations and mechanisms, which driven by the pandemic and supported by contextual factors, enabled the R&D paradigm shift and consequently made it possible to develop, manufacture, and deliver vaccines at a rapid pace. The cause-effect relationships among these elements are established in the second entity of the meta-model depicted in Fig. 3, known as the innovation causal model. In addition to cause-effect relationships underlying the R&D paradigm shift, the innovation causal model brings empirical evidence on the adoption of mechanisms from previous models, such as the rationale of pulled and pushed demand to sustain the R&D investments by Michael Kremer [25, 26] and Brogand and Mossialos [27]. Moreover, some innovations presented by the second entity of the meta-model empirically present themselves as countermeasures to the bottlenecks identified in other models [e.g., 31]. One example is the use of new vaccine platforms (e.g., DNA/RNA or recombinant viral vector) to reduce manufacturing complexity (M8) and facilitate their reproducibility and validation (M9) to the point of lowering costs (O4) and GMP lead time (O5). Another example includes the adoption of adaptive clinical trials (I6) combined with the rolling submission/review (I5) as a way to enable the execution of phases of clinical trials in parallel and thereby reduce clinical development and licensing lead time (O6 and O2).
Innovations, like the adoption of in silico clinical trials [e.g., 28], were not identified as stylized facts in the pandemic context and, consequently, are neither in the first nor in the second entity of the meta-model. However, they appear in the third entity, called the prospective framework (Table 2), as guidelines for future innovations. Besides bringing the guidelines in a single source, the prospective framework also classifies them according to their nature (e.g., technological, process, and infrastructure). By proposing the infrastructure category, the third entity of the meta-model expands the innovation classes offered by Defendi, Madeira, and Borschiver [4], which are primarily limited to the technological and process perspectives. In summary, the meta-model provides a holistic view of the vaccine R&D paradigm for pandemic contexts, which not only explains the mechanisms of change, but also brings guidelines for future changes, which is believed to be the main contribution of this work.
Closing Remarks
This work integrated the results of 27 studies into a meta-model for vaccine innovation in pandemic contexts, providing a framework for emergencies in which regulators, industry, governments, academia, and society face considerable challenges and must make difficult trade-off decisions. The meta-model proposed here combines three dimensions, each comprising specific elements related to one another in a cumulative causation dynamic. This helps to identify the key issues that need to be addressed at the onset of future outbreaks and to guide better policy and investment decisions for supporting faster, safer, and more effective vaccine development, manufacturing, and uptake in pandemic contexts.
In terms of limitations, some have been identified. The first is that the meta-model results from a joint evaluation of only 27 works, making it impossible to generalize statistically, but only in analytical terms. Another limitation is that the prospective framework presented in Table 2 limits itself to the indications of future directions recovered from the works comprising the corpus of analysis, thus leaving out the side effects of these indications over time and space. Concerning the fast-track vaccine R&D model outlined in Fig. 2, some stage-gates showed zero frequency, which may have left important innovations and mechanisms out of the meta-model. One of the hypotheses is that the granularity of the studies making up the corpus of analysis did not allow the identification of these stage-gates. This limitation could be overcome in future works by considering a corpus of analysis composed of longitudinal studies on each specific factor along the three dimensions of the model. Finally, immunization and vaccine uptake in a pandemic and the multifocal nature of related challenges are outside the boundaries of the meta-model built in this work. In this sense, models that expand these boundaries may create opportunities for future research, contributing to understanding the process from end to end.
Notes
In this work, the letter M of ‘monitoring’ was replaced by T of ‘tracking’ in order not to overlap with M of ‘mechanism’ stem from the CIMO-logic.
References
Pogostin BH, McHugh KJ. Novel vaccine adjuvants as key tools for improving pandemic preparedness. Bioengineering. 2021;8. https://doi.org/10.3390/bioengineering8110155.
Hatchett R, Lurie N. Outbreak response as an essential component of vaccine development. Lancet Infect Dis. 2019;19:e399–403. https://doi.org/10.1016/S1473-3099(19)30305-6.
Lurie N, Saville M, Hatchett R, Halton J. Developing covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969–73. https://doi.org/10.1056/NEJMp2005630.
Defendi HGT, Madeira L da S, Borschiver S. Analysis of the COVID-19 vaccine development process: an exploratory study of accelerating factors and innovative environments. J Pharm Innov. 2021. https://doi.org/10.1007/s12247-021-09535-8.
Newland M, Durham D, Asher J, Treanor JJ, Seals J, Donis RO, Johnson RA. Improving pandemic preparedness through better, faster influenza vaccines. Expert Rev Vaccines. 2021;20:235–42. https://doi.org/10.1080/14760584.2021.1886931.
González-Valdez J, Aguilar-Yáñez JM, Benavides J, Rito-Palomares M. DNA based vaccines offer improved vaccination supply for the developing world. J Chem Technol Biotechnol. 2013;88:979–82. https://doi.org/10.1002/jctb.4046.
Pagliusi S, Hayman B, Jarrett S. Vaccines for a healthy future: 21st DCVMN Annual General Meeting 2020 report. Vaccine. 2021;39(18):2479–88. https://doi.org/10.1016/j.vaccine.2021.03.025.
Giersing B, Shah N, Kristensen D, Amorij JP, Kahn AL, Gandrup-Marino K, Jarrahian C, Zehrung D, Menozzi-Arnaud M. Strategies for vaccine-product innovation: creating an enabling environment for product development to uptake in low- and middle-income countries. Vaccine. 2021;39:7208–19. https://doi.org/10.1016/j.vaccine.2021.07.091.
Plotkin SA, Orenstein WA, Offit PA, Edwards KM. Plotkin’s vaccines. 7th ed. Elsevier; 2018.
Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med. 2008;59:1–12. https://doi.org/10.1146/annurev.med.59.090506.155819.
Rafols I, Hopkins MM, Hoekman J, Siepel J, O’Hare A, Perianes-Rodríguez A, Nightingale P. Big Pharma, little science?. A bibliometric perspective on Big Pharma’s R&D decline. Technol Forecast Soc Change. 2014;81:22–38. https://doi.org/10.1016/j.techfore.2012.06.007.
Won J-H, Lee H. Can the covid‐19 pandemic disrupt the current drug development practices?. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms22115457.
Chary KV. Expedited drug review process: fast, but flawed. J Pharmacol Pharmacother. 2016;7:57–61. https://doi.org/10.4103/0976-500X.184768.
Jarow JP, Lurie P, Ikenberry SC, Lemery S. Overview of FDA’s expanded access program for investigational drugs. Ther Innov Regul Sci. 2017;51:177–9. https://doi.org/10.1177/2168479017694850.
Kepplinger EE. FDA’s expedited approval mechanisms for new drug products. Biotechnol Law Rep. 2015;34:15–37. https://doi.org/10.1089/blr.2015.9999.
FDA, Fast Track. 2018. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track. Accessed 15 Dec 2022.
Monrad JT, Sandbrink JB, Cherian NG. Promoting versatile vaccine development for emerging pandemics. NPJ Vaccines. 2021;6. https://doi.org/10.1038/s41541-021-00290-y.
Forman R, Shah S, Jeurissen P, Jit M, Mossialos E. COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy (New York). 2021;125:553–67. https://doi.org/10.1016/j.healthpol.2021.03.013.
Fialho B de C, Hasenclever L, Mello JMC The geography of innovation in the pharmaceutical industry: assessing implications for developing countries. In: Gibson D, Heitor M, Ibarra A, editors. Connect. People, Ideas Resour. Across Communities, Purdue University; 2007.
Borja Reis CFD, Pinto JPG. Center–periphery relationships of pharmaceutical value chains: a critical analysis based on goods and knowledge trade flows. Rev Polit Econ. 2022;34:124–45. https://doi.org/10.1080/09538259.2021.1882192.
Biswas K. The pharmaceutical value chain—an introduction. In: Pharma’s Prescr., Elsevier; 2014. p. 9–65. https://doi.org/10.1016/B978-0-12-407662-4.00002-7.
Wagner JA, Dahlem AM, Hudson LD, Terry SF, Altman RB, Gilliland CT, DeFeo C, Austin CP. Application of a dynamic map for learning, communicating, navigating, and improving therapeutic development. Clin Transl Sci. 2018;11:166–74. https://doi.org/10.1111/cts.12531.
Ribeiro C dos S, van Roode MY, Haringhuizen GB, Koopmans MP, Claassen E, van de Burgwal LHM. How ownership rights over microorganisms affect infectious disease control and innovation: a root-cause analysis of barriers to data sharing as experienced by key stakeholders. PLoS One. 2018;13. https://doi.org/10.1371/journal.pone.0195885.
Van de Burgwal LHM, Ribeiro CDS, Van der Waal MB, Claassen E. Towards improved process efficiency in vaccine innovation: the Vaccine Innovation Cycle as a validated, conceptual stage-gate model. Vaccine. 2018;36:7496–508. https://doi.org/10.1016/j.vaccine.2018.10.061.
Kremer M. Creating markets for new vaccines. Part I: Rationale. 2000. https://doi.org/10.1086/ipe.1.25056141.
Kremer M. Creating markets for new vaccines. Part II: Design Issues. 2000. https://doi.org/10.1086/ipe.1.25056142.
Brogan D, Mossialos E. Applying the concepts of financial options to stimulate vaccine development. Nat Rev Drug Discov. 2006;5:641–7. https://doi.org/10.1038/nrd2035.
De Groot AS, Einck L, Moise L, Chambers M, Ballantyne J, Malone RW, Ardito M, Martin W. Making vaccines “on demand”: a potential solution for emerging pathogens and biodefense? Hum Vaccin Immunother. 2013;9:1877–84. https://doi.org/10.4161/hv.25611.
Papaneri AB, Johnson RF, Wada J, Bollinger L, Jahrling PB, Kuhn JH. Middle East respiratory syndrome: obstacles and prospects for vaccine development. Expert Rev Vaccines. 2015;14:949–62. https://doi.org/10.1586/14760584.2015.1036033.
Saadatian-Elahi M, Bloom D, Plotkin S, Picot V, Louis J, Watson M. Vaccination ecosystem health check: achieving impact today and sustainability for tomorrow. BMC Proc. 2017;11:1–6. https://doi.org/10.1186/s12919-016-0069-y.
Drury G, Jolliffe S, Mukhopadhyay TK. Process mapping of vaccines: Understanding the limitations in current response to emerging epidemic threats. Vaccine. 2019;37:2415–21. https://doi.org/10.1016/j.vaccine.2019.01.050.
Ermel APC, Lacerda DP, Morandi MIWM, Gauss L. Literature reviews: modern methods for investigating scientific and technological knowledge. Springer International Publishing; 2021. https://doi.org/10.1007/978-3-030-75722-9.
Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, Davies P, Kleijnen J, Churchill R. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. https://doi.org/10.1016/j.jclinepi.2015.06.005.
Brunton G, Stansfield C, Thomas J. Finding relevant studies. In: An Introd. to Syst. Rev. London: Sage Publications Ltd; 2012. Chapter 5, pages 9-122, ISBN 978-1-4739-2942-5.
Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. ACM Int Conf Proceeding Ser. 2014. https://doi.org/10.1145/2601248.2601268.
Friede M, Palkonyay L, Alfonso C, Pervikov Y, Torelli G, Wood D, Kieny MP. WHO initiative to increase global and equitable access to influenza vaccine in the event of a pandemic: Supporting developing country production capacity through technology transfer. Vaccine. 2011;29:A2–7. https://doi.org/10.1016/j.vaccine.2011.02.079.
Miyaki C, Meros M, Precioso AR, Raw I. Influenza vaccine production for Brazil: a classic example of successful North-South bilateral technology transfer. Vaccine. 2011;29:A12–5. https://doi.org/10.1016/j.vaccine.2011.04.127.
Abelin A, Colegate T, Gardner S, Hehme N, Palache A. Lessons from pandemic influenza A(H1N1): the research-based vaccine industry’s perspective. Vaccine. 2011;29:1135–8. https://doi.org/10.1016/j.vaccine.2010.11.042.
Kieny M-P. Lessons learned from Ebola Vaccine R&D during a public health emergency. Hum Vaccin Immunother. 2018;14:2114–5. https://doi.org/10.1080/21645515.2018.1442161.
Graham JE. Ebola vaccine innovation: a case study of pseudoscapes in global health. Crit Public Health. 2019;29:401–12. https://doi.org/10.1080/09581596.2019.1597966.
Dinda AK, Tripathi SK, John B. Revisiting regulatory framework in India for accelerated vaccine development in pandemics with an evidence-based fast-tracking strategy. Indian J Med Res. 2020;152:156–63. https://doi.org/10.4103/ijmr.IJMR_3640_20.
Hanney SR, Wooding S, Sussex J, Grant J. From COVID-19 research to vaccine application: why might it take 17 months not 17 years and what are the wider lessons?. Heal Res Policy Syst. 2020;18. https://doi.org/10.1186/s12961-020-00571-3.
PwC, Pharma 2020: Virtual R&D Which path will you take?. 2020. https://www.pwc.com/gx/en/archive/industries/pharmaceuticals-life-sciences/pharma2020-virtual-rdwhich-path-will-you-take.html. Accessed 20 Feb 2022.
Lurie N, Keusch GT, Dzau VJ. Urgent lessons from COVID-19: why the world needs a standing, coordinated system and sustainable financing for global research and development. Lancet. 2021;397:1229–36. https://doi.org/10.1016/S0140-6736(21)00503-1.
McMillan G, Mayer C, Tang R, Liu Y, LaVange L, Antonijevic Z, Beckman RA. Planning for the next pandemic: ethics and innovation today for improved clinical trials tomorrow. Stat Biopharm Res. 2021. https://doi.org/10.1080/19466315.2021.1918236.
Lee Y, Ng M, Daniel K, Wayne E. Rapid growth in the COVID-19 era. MRS Bull. 2021;46:847–53. https://doi.org/10.1557/s43577-021-00185-2.
Aars OK, Clark M, Schwalbe N. Increasing efficiency in vaccine production: a primer for change. Vaccine X. 2021;8. https://doi.org/10.1016/j.jvacx.2021.100104.
Carneiro DC, Sousa JD, Monteiro-Cunha JP. The COVID-19 vaccine development: a pandemic paradigm. Virus Res. 2021;301. https://doi.org/10.1016/j.virusres.2021.198454.
Bloom DE, Cadarette D, Ferranna M, Hyer RN, Tortorice DL. How new models of vaccine development for COVID-19 have helped address an epic public health crisis. Health Aff (Millwood). 2021;40:410–8. https://doi.org/10.1377/hlthaff.2020.02012.
Knezevic I, Liu MA, Peden K, Zhou T, Kang H-N. Development of mRNA vaccines: scientific and regulatory issues. Vaccines. 2021;9:1–11. https://doi.org/10.3390/vaccines9020081.
Dresch A, Lacerda DP, Antunes JAV Jr. Design science research: a method for scientific and technology advancement. Springer; 2015. https://doi.org/10.1007/978-3-319-07374-3.
Denyer D, Tranfield D, van Aken JE. Developing design propositions through research synthesis. Organ Stud. 2008;29:249–69. https://doi.org/10.1177/0170840607088020.
Strauss A, Corbin J. Basics of qualitative research: grounded theory procedures and techniques. 2nd ed. Newbury Park: SAGE Publications; 1990.
Bardin L. L’analyse de contenu [Content Analysis]. Paris: Presses Universitaires de France Le Psychologue; 1993.
Quivy R. Manuel de Recherche en Sciences Sociales [Manual of Scientific Research in Social Science], Dunod, Paris, 1995. https://tecnologiamidiaeinteracao.files.wordpress.com/2018/09/quivy-manual-investigacao-novo.pdf. Accessed 22 Mar 2022.
Velho SRK, Simonetti ML, de Souza CRP, Ikegami MY. Nível de Maturidade Tecnológica: uma sistemática para ordenar tecnologias [Technological Maturity Level: a system for ordering technologies]. Parcerias Estratégicas. 2017;22:119–40.
WHO. Listings of WHO’s response to COVID-19. 2021. https://www.who.int/news/item/29-06-2020-covidtimeline. Accessed 28 Dec 2021.
AJMC. A Timeline of COVID-19 Developments in 2020. 2021. https://www.ajmc.com/view/a-timeline-of-covid19-developments-in-2020. Accessed 28 Dec 2021.
Yearworth M, White L. The uses of qualitative data in multimethodology: developing causal loop diagrams during the coding process. Eur J Oper Res. 2013;231:151–61. https://doi.org/10.1016/j.ejor.2013.05.002.
Cox JF, Schleier JG. Theory of constraints handbook, McGraw-Hill, New York; 2010. https://doi.org/10.1002/cbdv.200490137.
Lacerda DP, Rodrigues LH, da Silva AC. Evaluating the synergy of business process engineering and theory of constraints thinking process. Production. 2011;21:284–300. https://doi.org/10.1590/S0103-65132011005000019.
Walsh D, Downe S. Meta-synthesis method for qualitative research: a literature review. J Adv Nurs. 2005;50:204–11. https://doi.org/10.1111/j.1365-2648.2005.03380.x.
Noblit GW, Hare RD. Meta-ethnography: synthesizing qualitative studies. 1st ed. Newbury Park: SAGE Publications; 1988. https://doi.org/10.4135/9781412985000.
Eisenhardt KM. Building theories from case study research. Acad Manag Rev. 1989;14:532–50.
Cooper RG. Perspective: The stage-gate idea-to-launch process—update, what’s new, and nexgen systems. J Prod Innov Manag. 2008;25:213–32.
Acknowledgements
The authors appreciate the editor’s and the reviewers’ deep commitment, who provided in-depth and constructive criticism that has made it possible to improve the quality of our manuscript significantly. We also thank the Bio-Manguinhos/Fiocruz Institute of Immunobiological Technology for allowing this research to be conducted, as well as Coordination for the Improvement of Higher Education Personnel (CAPES) and the Brazilian National Council for Scientific and Technological Development (CNPq) for funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The literature review, analysis, and synthesis were performed by Beatriz C. Fialho and Leandro Gauss. The first draft of the manuscript was written by Beatriz C. Fialho and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for Publication
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing, we confirm that we have followed the regulations of our institutions concerning intellectual property.
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Vaccine Innovation Cycle [24]: a Defined stages and gates, which occur in a relatively predictable order and timing; b Undefined stages and gates, which their occurrence and timing are contingent on a wide variety of factors; c Monitoring stages and gates, which occur continuously and iteratively
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fialho, B.C., Gauss, L., Soares, P.F. et al. Vaccine Innovation Meta-Model for Pandemic Contexts. J Pharm Innov 18, 1145–1193 (2023). https://doi.org/10.1007/s12247-023-09708-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09708-7