Abstract
Purpose
Poor oral absorption, low bioavailability, short half-life, and gastrointestinal effects due to high dose of metformin required in the management of type-2 diabetes mellitus have spurred researchers to pay greater attention to the development of novel drug delivery systems to tackle these challenges. The aim of this study was to formulate and evaluate sunseed-oil-based PEGylated nanostructured lipid carriers (PEG-NLC) for enhanced delivery and prolonged antidiabetic activity of metformin.
Methods
The PEG-NLC and non-PEGylated NLC were formulated by high shear homogenization and thereafter characterized by scanning electron microscopy, mean particle size determination, photon correlation spectroscopy, differential scanning calorimetry (DSC), and Fourier transform infrared (FT-IR) spectroscopy. In vitro drug release, pharmacodynamic studies using alloxanized rat model, pharmacokinetics and safety evaluations were carried out. Results were compared with those of controls (market and pure samples of metformin).
Results
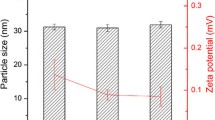
DSC results showed reduced crystallinity and hence greater possibility of enhanced drug solubility and entrapment, while FTIR results showed drug-excipient compatibility. The PEG-NLCs were safe, were stable spherical nanoparticles, had mean particle size, polydispersity indices and zeta potentials in the range of 290.6–880.6 nm, 0.494–0.625, and 26.1–32.8 mV, respectively. The PEG-NLCs showed enhanced drug release in simulated biorelevant media and prolonged antidiabetic activity compared with both non-PEGylated NLC and controls. Batch D40 containing the highest amount of PEG-4000 (optimized formulation) gave sixfold increase in pharmacokinetics properties than marketed sample (Glucophage®).
Conclusion
Sunseed-oil-based PEGylated NLC has proven to be a stable and safe carrier system for enhanced delivery and prolonged antidiabetic activity of metformin.



















Similar content being viewed by others
References
Shi F, Wei Z, Zhao Y, Xu X. Nanostructured lipid carriers loaded with baicalin: An efficient carrier for enhanced antidiabetic effects. Pharmacogn Mag. 2016;12(47):198–202.
Heydari I, Radi V, Razmjou S, Amiri A. Chronic complications of diabetes mellitus in newly diagnosed patients. Int J Diabet Mellitus. 2010;2(1):61–3.
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
Suvi K, Belma M, Pouya S, Paraskevi S, editors. IDF Diabetes Atlas. 9th ed. [Internet]. International Diabestes Federation; 2019. Available from: http://www.diabetesatlas.org. Accessed 14 Jun 2020.
Momoh M, Kenechukwu F, Attama A. Formulation and evaluation of novel solid lipid microparticles as a sustained release system for the delivery of metformin hydrochloride. Drug Deliv. 2013;20(3–4):102–11.
Momoh M, Adikwu M, Ibezim E, Attama A. Effect of metformin and Vernonia amygdalina leaf extract loaded PEGylated-mucin formulation on haematological, kidney and liver indices of healthy and diabetes rats. J Pharm Res. 2011;4(10):3455–9.
Shukla SK, Kulkarni NS, Chan A, Parvathaneni V, Farrales P, Muth A, et al. Metformin-encapsulated liposome delivery system: An effective treatment approach against breast cancer. Pharmaceutics. 2019;11(11). http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6920889/. Accessed 14 Jun 2020.
Momoh M, Adedokun M, Adikwu M, Kenechukwu F, Ibezim E, Ugwoke E. Design, characterization and evaluation of PEGylated-mucin for oral delivery of metformin hydrochloride. Afr J Pharm Pharmacol. 2013;7(7):347–55.
Kumar S, Bhanjana G, Verma RK, Dhingra D, Dilbaghi N, Kim K-H. Metformin-loaded alginate nanoparticles as an effective antidiabetic agent for controlled drug release. J Pharm Pharmacol. 2017;69(2):143–50.
Bhujbal S, Dash AK. Metformin-loaded hyaluronic acid nanostructure for oral delivery. AAPS Pharm Sci Tech. 2018;19(6):2543–53.
Santhosh C, Deivasigamani K, Venkata R. Enhanced effects of metformin loaded chitoson nanoparticles in L6 myotubes: In vitro evaluation. Pharm Lett. 2017;9(7):48–63.
Sahu AK, Verma A. Development and statistical optimization of chitosan and eudragit based gastroretentive controlled release multiparticulate system for bioavailability enhancement of metformin HCl. J Pharm Investig. 2016;46(3):239–52.
Hasan AA, Madkor H, Wageh S. Formulation and evaluation of metformin hydrochloride-loaded niosomes as controlled release drug delivery system. Drug Delivery. 2013;20(3–4):120–6.
Rostamkalaei SS, Akbari J, Saeedi M, Morteza-Semnani K, Nokhodchi A. Topical gel of metformin solid lipid nanoparticles: A hopeful promise as a dermal delivery system. Colloids Surf, B. 2019;175:150–7.
Sharma R, Sharma N, Rana S, Shivkumar H. Solid lipid nanoparticles as a carrier of metformin for transdermal delivery. Int J Drug Deliv. 2013;5:137–45.
Adhikari P, Pal P, Das AK, Ray S, Bhattacharjee A, Mazumder B. Nano lipid-drug conjugate: An integrated review. Int J Pharm. 2017;529(1–2):629–41.
Attama A, Momoh M, Builders P. Lipid nanoparticulate drug delivery systems: A revolution in dosage form design and development. In: Sezer AD, editor. Recent Advances in Novel Drug Carrier Systems [Internet]. Rijeka,Croatia: InTech; 2012. p. 107–40. Available from: http://www.intechopen.com/books/recent-advances-in-novel-drug-carrier-systems/lipid-nanoparticulate-drug-delivery-systems-a-revolution-in-dosage-form-design-and-development. Accessed 9 Jun 2021.
Chime SA, Onyishi IV. Lipid-based drug delivery systems (LDDS): Recent advances and applications of lipids in drug delivery. Afr J Pharm Pharmacol. 2013;7(48):3034–59.
Beloqui A, Solinís MÁ, Rodríguez-Gascón A, Almeida AJ, Préat V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine. 2016;12(1):143–61.
Doktorovová S, Kovačević Ab, Garcia Ml, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2016;108. Available from: https://pubmed.ncbi.nlm.nih.gov/27519829/ [cited 30 Jan 2022]
Carbone C, Teixeira MD, Sousa MD, Martins-Gomes C, Silva AM, Souto EM, et al. Clotrimazole-loaded Mediterranean essential oils NLC: A synergic treatment of candida skin infections. Pharmaceutics. 2019;11(5):231.
Teixeira MC, Carbone C, Souto EB. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog Lipid Res. 2017;68:1–11.
Severino P, Andreani T, Macedo AS, Fangueiro JF, Santana MHA, Silva AM, et al. Current state-of-art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J Drug Deliv. 2012;12: 750891.
Doktorovova S, Shegokar R, Souto E. Role of excipients in formulation development and biocompatibility of lipid nanoparticles (SLNs/NLCs). In: Grumezescu A, editor. Nanostructures for novel therapy. Amsterdam, The Netherlands: Elsevier; 2017. p. 811–43.
Pardeike J, Hommoss A, Müller Rh. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366(1–2):170–84.
Zhang T, Chen J, Zhang Y, Shen Q, Pan W. Characterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposide. Eur J Pharm Sci. 2011;43(3):174–9.
Nnamani PO, Hansen S, Windbergs M, Lehr C-M. Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application. Int J Pharm. 2014;477(1–2):208–17.
Jaiswal P, Gidwani B, Vyas A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artificial Cells, Nanomedicine, and Biotechnology. 2016;44(1):27–40.
Das S, Ng WK, Tan RBH. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci. 2012;47(1):139–51.
Iqbal MA, Md S, Sahni Jk, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Target. 2012;20(10). Available from: https://pubmed.ncbi.nlm.nih.gov/22931500/ [cited 30 Jan 2022]
Kaur K, Nautiyal U, Singh D. Nanostructured lipid carrier for bioavailability enhancement. Int J Recent Adv Sci Technol. 2015;2:1–7.
Fang C-L, Al-Suwayeh SA, Fang J-Y. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat Nanotechnol. 2013;7(1):41–55.
Rajalakshmi G, Dhanapal CK, Sundhararajan R. An insight to nanostructured lipid carrier system. J Drug Deliv Therap. 2020;10:173–82.
Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10(21). Available from: https://pubmed.ncbi.nlm.nih.gov/16243265/ [cited 30 Jan 2022]
Kenechukwu FC, Attama AA, Ibezim EC, Nnamani PO, Umeyor CE, Uronnachi EM, et al. Novel intravaginal drug delivery system based on molecularly PEGylated lipid matrices for improved antifungal activity of miconazole nitrate. BioMed Res Int. 2018;20:1–18.
van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14(11):1007–17.
Chen Q, Liu G, Liu S, Su H, Wang Y, Li J, et al. Remodeling the tumor microenvironment with emerging nanotherapeutics. Trends Pharmacol Sci. 2018;39(1):59–74.
Luo C, Sun J, Du Y, He Z. Emerging integrated nanohybrid drug delivery systems to facilitate the intravenous-to-oral switch in cancer chemotherapy. J Control Rel. 2014;176:94–103.
Nag OK, Awasthi V. Surface engineering of liposomes for stealth behavior. Pharmaceutics. 2013;5(4):542–69.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51.
Dai Y, Xing H, Song F, Yang Y, Qiu Z, Lu X, et al. Biotin-conjugated multilayer poly [D, L-lactide-co-glycolide]-lecithin-polyethylene glycol nanoparticles for targeted delivery of doxorubicin. J Pharm Sci. 2016;105(9):2949–58.
Barenholz Y, Anselem S. Quality control assays in the development and clinical use of liposome-based formulation. In Gregoriades G (ed): CRC Press, Boca Raton, FL; 1993. p. 527–616.
Eldridge J, Staas J, Meulbroek J, McGhee J, Tice T, Gilley R. Biodegradable microspheres as a vaccine delivery system. Mol immunol. 1991;28:287–94.
Pingale A, Gondkar S, Saudagar R. Nanostructured lipid carrier (NLC): A modern approach for intranasal drug delivery. World J Pharm Res. 2018;7(9):1574–88.
Uner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharmazie. 2005;61:375–86.
Reginald-Opara JN, Attama A, Ofokansi K, Umeyor C, Kenechukwu F. Molecular interaction between glimepiride and Soluplus® -PEG 4000 hybrid based solid dispersions: Characterisation and anti-diabetic studies. Int J Pharm. 2015;496(2):741–50.
Kerc J, Srcic S. Thermal analysis of glassy pharmaceuticals. Thermochim Acta. 1995;248:81–95.
Attama AA, Nkemnele MO. In vitro evaluation of drug release from self micro-emulsifying drug delivery systems using a biodegradable homolipid from Capra hircus. Int J Pharm. 2005;304(1–2):4–10.
Uronnachi E, Ogbonna J, Kenechukwu F. Formulation and release characteristics of zidovudine-loaded solidified lipid microparticles. Trop J Pharm Res. 2014;13(2):199–204.
Uronnachi E, Ogbonna J, Kenechukwu F, Mumuni M, Attama A, Okore V. Pharmacokinetics and biodistribution of zidovudine loaded in a solidified reverse micellar delivery system. Int J Drug Deliv. 2013;5:73–80.
Tsutsumi S, Iida M, Tada N, Kojima T, Ikeda Y, Moriwaki T, et al. Characterization and evaluation of miconazole salts and cocrystals for improved physicochemical properties. Int J Pharm. 2011;421(2):230–6.
Kamboj VK, Verma PK. Preparation and characterization of metformin-loaded stearic acid coupled F127 nanoparticles. Asian J Pharm Clin Res. 2018;11(8):212–7.
Javidfar S, Pilehvar-Soltanahmadi Y, Farajzadeh R, Lotfi-Attari J, Shafiei-Irannejad V, Hashemi M, et al. The inhibitory effects of nano-encapsulated metformin on growth and hTERT expression in breast cancer cells. J Drug Deliv Sci Technol. 2018;43:19–26.
Sharma G, Parchur AK, Jagtap JM, Hansen CP, Joshi A. Hybrid nanostructures in targeted drug delivery. In: Hybrid Nanostructures for Cancer Theranostics. Elsevier; 2019. p. 139–58. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128139066000081. Accessed 26 Feb 2021.
Wadher KJ, Kakde RB, Umekar MJ. Development of a sustained-release tablet of metformin hydrochloride containing hydrophilic eudragit and ethyl cellulose polymer. Int J Clin Pharm. 2011;2(5):1–6.
Weng J, Tong HHY, Chow SF. In vitro release study of the polymeric drug nanoparticles: Development and validation of a novel method. Pharmaceutics. 2020;12(8):732.
Cardot J, Beyssac E, Alric M. In vitro–in vivo correlation: Importance of dissolution in IVIVC. Dissolution Technologies. 2007;14.
Sjögren E, Abrahamsson B, Augustijns P, Becker D, Bolger M, Brewster M, et al. In vivo methods for drug absorption - Comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur J Pharm Sci. 2014;57:23–31.
Bruschi ML, editor. Mathematical models of drug release. In: Strategies to Modify the Drug Release from Pharmaceutical Systems. Woodhead Publishing; 2015. p. 63–86. Available from: https://www.sciencedirect.com/science/article/pii/B9780081000922000059. Accessed 12 Aug 2019.
Khan MA, Shefeeq T. Role of mathematical modeling in controlled drug delivery. J Sci Res. 2009;1(3):539–50.
Permanadewi I, Kumoro A, Wardhani D, Aryanti N. Modelling of controlled drug release in gastrointestinal tract simulation. J Phys: Conf Ser. 2019;1295:1–8.
Siepmann J, Peppas N. Higuchi equation: Derivation, applications, use and misuse. Int J Pharm. 2011;418:6–12.
Ngwuluka NC, Kotak DJ, Devarajan PV. Design and characterization of metformin-loaded solid lipid nanoparticles for colon cancer. AAPS PharmSciTech. 2017;18(2):358–68.
Xu Q, Zhu T, Yi C, Shen Q. Characterization and evaluation of metformin-loaded solid lipid nanoparticles for celluar and mitochondrial uptake. Drug Dev Ind Pharm. 2016;42(5):701–6.
Mishra A, Imam SS, Aqil M, Ahad A, Sultana Y, Ameeduzzafar, et al. Carvedilol nano lipid carriers: formulation, characterization and in-vivo evaluation. Drug Deliv. 2016;23(4):1486–94.
Shi L, Zhang J, Zhao M, Tang S, Cheng X, Zhang W, et al. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale. 2021;13(24):10748–64.
Hashizaki K, Taguchi H, Itoh C, Sakai H, Abe M, Saito Y, et al. Effects of poly(ethylene glycol) (PEG) concentration on the permeability of PEG-grafted liposomes. Chem Pharm Bull. 2005;53(1):27–31.
Edwards K, Almgren M. Surfactant-induced leakage and structural change of lecithin vesicles: effect of surfactant headgroup size. Langmuir. 1992;8(3):824–32.
Nicholas A., Scott M, Kennedy N, Jones M. Effect of grafted PEG-2000 on the size and permeability of vesicles. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1996;1304(2):120–8.
De Leo V, Ruscigno S, Trapani A, Di Gioia S, Milano F, Mandracchia D, et al. Preparation of drug-loaded small unilamellar liposomes and evaluation of their potential for the treatment of chronic respiratory diseases. Int J Pharm. 2018;545(1–2):378–88.
Lin T-T, Gao D-Y, Liu Y-C, Sung Y-C, Wan D, Liu J-Y, et al. Development and characterization of sorafenib-loaded PLGA nanoparticles for the systemic treatment of liver fibrosis. J Control Rel. 2016;221:62–70.
Gadad AP, Tigadi SG, Dandagi PM, Mastiholimath VS, Bolmal UB. Rosuvastatin-loaded nanostructured lipid carrierj for enhancement of oral bioavailability. Indian J Pharm Ed Res. 2016;50(4):605–11.
Funding
This research work received financial support from the Tertiary Education Trust Fund (TETFund) (Grant no. TETFUND/DR&D/CE/NRF/2019/STI/46/) by Government of Nigeria. Dr. Franklin C. Kenechukwu wishes to acknowledge Phospholipid GmbH, Köln, Germany, for generous provision of Phospholipon® 90H (P90H), Ph. Eur. Carl Roth GmbH + Co. KG Karlsruhe, Germany, for the king gift of polyethylene glycol 4000 (PEG 4000) and beeswax.
Author information
Authors and Affiliations
Contributions
Franklin Chimaobi Kenechukwu did conceptualization, supervision, methodology, validation, resources, funding acquisition, writing—original and draft as well as revision; Daniel Okwudili Nnamani performed methodology, writing—original and draft as well as revision; Bright Ugochukwu Nmesirionye was involved in methodology, formal analysis, investigation, writing—review and editing; God’spower Tochukwu Isaac contributed to methodology and writing—review and editing; Mumuni Audu Momoh and Anthony Amaechi Attamadone methodology, validation, resources, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experimental protocols were conducted with strict adherence to the guidelines of the Institutional Animal Care and Use Committee of the University of Nigeria, Nsukka. Ethical clearance approval for in vivo antidiabetic studies was sought and obtained from the Faculty of Pharmaceutical Sciences Research Ethics Committee (UNN/FPS/2019-2020_017X) before the commencement of the in vivo animal studies.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kenechukwu, F.C., Nnamani, D.O., Nmesirionye, B.U. et al. PEGylated Lipid Nanocontainers Tailored with Sunseed-Oil-Based Solidified Reverse Micellar Solution for Enhanced Pharmacodynamics and Pharmacokinetics of Metformin. J Pharm Innov 18, 437–460 (2023). https://doi.org/10.1007/s12247-022-09654-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09654-w