Abstract
Purpose
Clofazimine displays high intersubject variability in absorption after oral administration because of its extremely low water solubility and high lipophilicity. The present investigation was aimed to prepare self micro emulsifying drug delivery system (SMEDDS) of clofazimine for improving its dissolution properties by using design of experiment (DoE) approach.
Methods
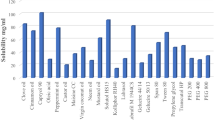
Various lipid, surfactant, and co-surfactants were screened based on their ability to solubilize clofazimine and their ability to make a clear transparent microemulsion upon dilution with water. Based on these tests, Capmul MCM, Tween-20, and Labrasol were selected as lipid, surfactant, and co-surfactant respectively. The design batches were prepared according to simplex lattice design and the optimized batch was selected by using the desirability function of design expert software. Contour plots and response surface plots were generated to understand the effect changed in formulation composition on critical product parameters (globule size and cumulative percentage drug release in 30 min).
Results
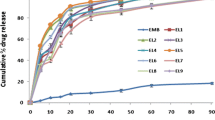
All the prepared batches released more than 85% drug in less than 60 min in 1.2 pH HCl and 6.8 Phosphate buffer. The validity of the model was proved by observed close agreement between the experimentally obtained values for optimized batch and predicted values given by the polynomial equation.
Conclusion
The present investigation successfully demonstrated the effectiveness of the simplex lattice design and desirability function for optimizing the SMEDDS formulation and its ability to explain the effects of formulation variables on critical product attributes.








Similar content being viewed by others
References
Suzuki K, Akama T, Kawashima A, Yoshihara A, Yotsu RR, Ishii N. Current status of leprosy: epidemiology, basic science and clinical perspectives. J Dermatol. 2012;39(2):121–9. https://doi.org/10.1111/j.1346-8138.2011.01370.x.
Browne SG, Hogerzeil LM. “B 663” in the treatment of leprosy. Preliminary report of a pilot trial. Lepr Rev. 1962;33:6–10.
Rodrigues LC, Lockwood DN. Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect Dis. 2011;11(6):464–70.
Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R. Clofazimine: current status and future prospects. J Antimicrobial Chemother. 2011:dkr444.
Murashov MD, LaLone V, Rzeczycki PM, Keswani RK, Yoon GS, Sud S, et al. The physicochemical basis of Clofazimine-induced skin pigmentation. J Invest Dermatol. 2018;138(3):697–703. https://doi.org/10.1016/j.jid.2017.09.031.
Garrelts JC. Clofazimine: a review of its use in leprosy and Mycobacterium avium complex infection. Dicp. 1991;25(5):525–31.
Holdiness M. Clinical pharmacokinetics of clofazimine. Clin Pharmacokinet. 1989;16(2):74–85. https://doi.org/10.2165/00003088-198916020-00002.
Narang AS, Srivastava AK. Evaluation of solid dispersions of clofazimine. Drug Dev Ind Pharm. 2002;28(8):1001–13.
Nie H, Su Y, Zhang M, Song Y, Leone A, Taylor LS, et al. Solid-state spectroscopic investigation of molecular interactions between clofazimine and hypromellose phthalate in amorphous solid dispersions. Mol Pharm. 2016;13(11):3964–75.
Zhang Y, Feng J, McManus SA, Lu HD, Ristroph KD, Cho EJ, et al. Design and solidification of fast-releasing clofazimine nanoparticles for treatment of cryptosporidiosis. Mol Pharm. 2017;14(10):3480–8.
Patel DP, Li P, Serajuddin AT. Enhanced microemulsion formation in lipid-based drug delivery systems by combining mono-esters of mediumchain fatty acids with di-or tri-esters. J Excipients Food Chem. 2016;3(2):1113.
Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv Drug Deliv Rev. 1997;25(1):47–58.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’drug delivery systems. Eur J Pharm Sci. 2000;11:S93–S8.
Dahan A, Hoffman A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J Control Release. 2008;129(1):1–10. https://doi.org/10.1016/j.jconrel.2008.03.021.
Weissman SA, Anderson NG. Design of experiments (DoE) and process optimization. A review of recent publications. Org Process Res Dev. 2014;19(11):1605–33.
Salazar J, Heinzerling O, Müller RH, Möschwitzer JP. Process optimization of a novel production method for nanosuspensions using design of experiments (DoE). Int J Pharm. 2011;420(2):395–403.
Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H, et al. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371(1):148–55.
Singh AK, Chaurasiya A, Singh M, Upadhyay SC, Mukherjee R, Khar RK. Exemestane loaded self-microemulsifying drug delivery system (SMEDDS): development and optimization. AAPS PharmSciTech. 2008;9(2):628–34.
Bandivadeka MM, Pancholi SS, Kaul-Ghanekar R, Choudhari A, Koppikar S. Self-microemulsifying smaller molecular volume oil (Capmul MCM) using non-ionic surfactants: a delivery system for poorly water-soluble drug. Drug Dev Ind Pharm. 2012;38(7):883–92.
Kumar S, Bhargava D, Thakkar A, Arora S. Drug carrier systems for solubility enhancement of BCS class II drugs: a critical review. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2013;30(3).
Misic Z, Šišak Jung D, Sydow G, Kuentz M. Understanding interactions of oleic acid with basic drugs in solid lipids on different biopharmaceutical levels. J Excipients Food Chem. 2014;(2):113–34 %V 5.
Prajapati HN, Dalrymple DM, Serajuddin AT. A comparative evaluation of mono-, di-and triglyceride of medium chain fatty acids by lipid/surfactant/water phase diagram, solubility determination and dispersion testing for application in pharmaceutical dosage form development. Pharm Res. 2012;29(1):285–305.
Craig D, Barker S, Banning D, Booth S. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int J Pharm. 1995;114(1):103–10.
Balakrishnan P, Lee B-J, Oh DH, Kim JO, Lee Y-I, Kim D-D, et al. Enhanced oral bioavailability of coenzyme Q10 by self-emulsifying drug delivery systems. Int J Pharm. 2009;374(1–2):66–72.
Lu J-L, Wang J-C, Zhao S-X, Liu X-Y, Zhao H, Zhang X, et al. Self-microemulsifying drug delivery system (SMEDDS) improves anticancer effect of oral 9-nitrocamptothecin on human cancer xenografts in nude mice. Eur J Pharm Biopharm. 2008;69(3):899–907. https://doi.org/10.1016/j.ejpb.2008.02.023.
Zhao L, Zhang L, Meng L, Wang J, Zhai G. Design and evaluation of a self-microemulsifying drug delivery system for apigenin. Drug Dev Ind Pharm. 2013;39(5):662–9.
Marinova KG, Alargova RG, Denkov ND, Velev OD, Petsev DN, Ivanov IB, et al. Charging of oil−water interfaces due to spontaneous adsorption of hydroxyl ions. Langmuir. 1996;12(8):2045–51. https://doi.org/10.1021/la950928i.
Shinoda K, Arai H. The correlation between phase inversion temperature in emulsion and cloud point in solution of nonionic emulsifier. J Phys Chem. 1964;68(12):3485–90. https://doi.org/10.1021/j100794a007.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhaval, M., Panjwani, M., Parmar, R. et al. Application of Simple Lattice Design and Desirability Function for Formulating and Optimizing SMEDDS of Clofazimine. J Pharm Innov 16, 504–515 (2021). https://doi.org/10.1007/s12247-020-09468-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09468-8