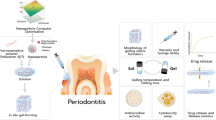
Abstract
Purpose
This study aims to formulate and evaluate the temperature-sensitive in situ gel of 0.5% (w/v) azithromycin (AZM) containing cyclodextrin inclusion complex by the cold method.
Method
To increase its aqueous solubility, it was incorporated as an inclusion complex in a 1:1 M ratio with hydroxypropyl β-cyclodextrin (HPβ-CD). The central composite design was used to optimize the effect of poloxamer 407 and Carbopol 934P (independent factors) on dependent factors such as percentage drug release at first hour (t50% and t90%), gelation temperature, and viscosity. Both the independent factors had a significant effect on the entire five response factors (p < 0.05). Each formulation was evaluated for clarity, pH, spreadability, syringeability, gelation strength, mucoadhesive property, in vitro drug release, gelation temperature, viscosity, and rheology.
Result
Based on maximum desirability, formulation containing 18.91% (w/v) of poloxamer 407 and 0.353% (w/v) of Carbopol 934P was considered an optimized batch. The value of percentage relative error exhibited a close agreement between observed and predicted values calculated using regression equations. Further, the optimized formulation showed a good drug release for a period of 54 h, which was more effective in the treatment of periodontal disease.
Conclusion
Thus, azithromycin (AZM) can be successfully formulated as a thermosensitive in situ mucoadhesive gel containing HPβ-CD for periodontal drug delivery.















Similar content being viewed by others
References
Saglie R, Newman MG, Carranza FA, Pattison GL. Bacterial invasion of gingiva in advanced periodontitis in humans. J Periodontol. 1982;53:217–22.
Adriaens PA, Jan A, Boeveri D, Loesche WJ. Bacterial invasion in root cementum and radicular dentin of periodontally diseased teeth in humans. J Periodontol. 1988;59:222–30.
Nair SC, Anoop KR. Intraperiodontal pocket: an ideal route for local antimicrobial drug delivery. J Adv Pharm Technol Res. 2012;3:9–15.
Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1994;4(1):1–6.
Greenstein G. Local drug delivery in the treatment of periodontal diseases: assessing the clinical significance of the results. J Periodontol. 2006;77:565–78.
Muniz FW, de Oliveira CC, Carvalho R, Moreira MM, de Moraes ME, Martins RS. Azithromycin: a new concept in adjuvant treatment of periodontitis. Eur J Pharmacol. 2013;705:135–9.
Pradeep AR, Bajaj P, Agarwal E, Rao NS, Naik SB, Kalra N, et al. Local drug delivery of 0.5% azithromycin in the treatment of chronic periodontitis among smokers. Aust Dent J. 2013;58:34–40.
Wadhwa S, Singhal S, Rawat S. In vitro dissolution enhancement of azithromycin in solid dispersion with PEG 6000 and β-CD. J Pharm Biomed Sci. 2016;06:551–6.
Idkaidek NM, Najib N, Salem I, Jilani J. Physiologically-based IVIVC of azithromycin. Am J Phramacol Sci. 2014;2:100–2.
Tiwari G, Tiwari R, Rai AK. Cyclodextrins in delivery systems: applications. J Pharm Bioallied Sci. 2010;2:72–9.
Gould S, Scott RC. 2-Hydroxypropyl-b-cyclodextrin (HP-b-CD): a toxicology review. Food Chem Toxicol. 2005;43:1451–9.
Castronuovo G, Niccoli M. Thermodynamics of inclusion complexes of natural and modified cyclodextrins with propranolol in aqueous solution at 298 K. Bioorg Med Chem. 2006;14:3883–7.
Pitcher GR, Newman HN, Strahan JD. Access to subgingival plaque by disclosing agents using mouth rinsing and direct irrigation. J Clin Periodontol. 1980;7(4):300–8.
Samaranayake L, Ferguson M. Delivery of antifungal agents to the oral cavity. Adv Drug Deliv Rev. 1994;13(2):161.
Ishida M, Nambu N, Nagai T. New mucosal dosage form of insulin. Chem Pharm Bull. 1982;29(3):980.
Collins AE, Deasy PB, MacCarthy DJ, Shanley DB. Evaluation of controlled release compact containing tetracycline hydrochloride bonded to tooth for the treatment of periodontal disease. Int J Pharm. 1989;51(2):103.
Elkayam R, Friedman M, Stabholz A, Soskolne AW, Sela MN, Golub L. Sustained release device containing minocycline for local treatment of periodontal disease. J Control Release. 1988;7(3):231.
Zhang W, Xu W, Ning C, Li M, Zhao G, Jiang W, et al. Long-acting hydrogel/microsphere composite sequentially releases dexmedetomidine and bupivacaine for prolonged synergistic analgesia. Biomaterials. 2018;18:30536–2.
Zhang W, Xu W, Ning C, Li M, Zhao G, Jiang W, et al. Precision-guided long-acting analgesia by gel-immobilized bupivacaine-loaded microsphere. Theranostics. 2018;8:3331–47.
Liua L, Fengb X, Peia Y, Wanga J, Dingb J, Chena L. α-Cyclodextrin concentration-controlled thermo-sensitive supramolecular hydrogels. Mater Sci Eng C. 2018;82:25–8.
Wei L, Chen J, Zhao S, Ding J, Chen X. Thermo-sensitive polypeptide hydrogel for locally sequential delivery of two-pronged antitumor drugs. Acta Biomater. 2017;53:30345–8.
Zhang Y, Ding J, Sun D, Sun H, Zhuang X, Chang F, et al. Thermogel-mediated sustained drug delivery for in situ malignancy chemotherapy. Mater Sci Eng C. 2015;49:262–8.
Mohanty D, Bakshi V, Simharaju N, Haque MA and Sahoo CK: A review on in-situ gel: a novel drug delivery system. Int J of Pharm Sci Rev and Res 2018; 50: 175-81.
Garala K, Joshi P, Shah M, Ramkishan A, Patel J. Formulation and evaluation of periodontal in situ gel. Int J Pharm Investig. 2013;3:29–41.
Miyazaki S, Endo K, Kawasaki N, Kubo W, Watanabe H, Attwood D. Oral sustained delivery of paracetamol from in situ gelling xyloglucan formulations. Drug Dev Ind Pharm. 2003;29:113–9.
Rajinikanth PS, Mishra B. Floating in situ gelling system of acetohydroxamic acid for clearance of H. pylori. Drug Dev Ind Pharm. 2008;34:577–87.
Arunachalam R, Rajeev V, Vedam V, Ganapathy S, Dhanavel J. Perioceutics in the management of periodontal disease. J Young Pharm. 2017;9(1):8–13.
Higuchi T, Connors KA. Phase solubility technique. Adv Anal Chem Instrum. 1965;4:117–212(1965).
Anshu S, Jain CP. 2013. Carvedilol-β-cyclodextrin systems: preparation, characterization and in vitro evaluation. J Pharm Sci. 2013;12:51–8.
Fangfang X, Qiuxia Y, Lilan W, Rui Q, Yunshan W, Yucui L, et al. Investigation of inclusion complex of patchouli alcohol with β-cyclodextrin. PLoS One. 2017;12:1–10.
Sivasubramanian L, Marvin MA, Jayashanker L, Ramu P, Raja TK. Visible spectrophotometric method for the determination of azithromycin in tablet. Indian J Pharm Sci. 2004:66;249–51.
Bhandwalkar MJ, Avachat AM. Thermoreversible nasal in situ gel of venlafaxine hydrochloride: formulation, characterization, and pharmacodynamic evaluation. AAPS PharmSciTech. 2013;14(1):101–10.
Singh RP, Kumar A, Pathak K. Thermally triggered mucoadhesive in situ gel of loratadine: β-cyclodextrin complex for nasal delivery. AAPS PharmSciTech. 2013;14:412–24.
M. Bansala, N. Mittala, S. K. Yadavb, G. Khanb, P. Gupta, B. Mishrab, G. Nath, Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: preparation, in-vitro characterization and antimicrobial study, J Oral Biol Craniofac Res 2018;8;126:133.
Dabhi MR, Nagori SA, Gohel MC, Parikh RK, Sheth NR. Formulation development of smart gel periodontal drug delivery system for local delivery of chemotherapeutic agents with application of experimental design. Drug Deliv. 2010;17:520–31.
Chaudhary B, Verma S. Preparation and evaluation of novel in situ gels containing acyclovir for the treatment of oral herpes simplex virus infections. Sci World J. 2014;3;1–8.
Galgatte UC, Kumbhar AB, Chaudhari PD. Development of in situ gel for nasal delivery: design, optimization, in vitro and in vivo evaluation. Drug Deliv. 2014;21:62–73.
)Dabhi MR, Sheth NR. Formulation development of physiological environment responsive periodontal drug delivery system for local delviery of metronidazole benzoate. Drug Dev Ind Pharm. 2013;39:425-36.
Altuntaş E, Yener G. Formulation and evaluation of thermoreversible in situ nasal gels containing mometasone furoate for allergic rhinitis. AAPS PharmSciTech. 2017.
Nasra MA, Khiri HM, Hazzah HA, Abdallah OY. Formulation, in-vitro characterization and clinical evaluation of curcumin in-situ gel for treatment of periodontitis. Drug Deliv. 2017;24:133–42.
Mahajan HS, Shah SK, Sanjay J, Surana SJ. Nasal in situ gel containing hydroxy propyl b-cyclodextrin inclusion complex of artemether: develeopment and in vitro evaluation. J Incl Phenom Macrocycl Chem. 2011;70:49–58.
Majithiya RJ, Ghosh PK, Umrethia ML, Murthy RS. Thermoreversible mucoadhesive gel fo nasal delivery of sumatriptan. AAPS PharmSciTech. 2006;7(3):67.
Abd AM, Razek E, Hasan AA, Sabry SA, Mahdy MA, Hamed EE. Metoclopramide hydrochloride thermally sensitive rectal in situ gelling system, a novel out-patient treatment for vomiting in pediatric age. J Drug Deliv Sci Tec. 2019:31;203–6.
Hea Z, Wanga Z, Zhanga H, Pana X, Sud W, Liangd D, et al. Doxycycline and hydroxypropyl-b-cyclodextrin complexin poloxamer thermalsensitive hydrogel for ophthalmic delivery. Acta Pharm Sin B. 2011;4:254–60.
Patel N, Thakkar V, Metalia V, Baldaniya L, Gandhi T, Gohel M. Formulation and development of ophthalmic in situ gel for the treatment ocular inflammation and infection using application of quality by design concept. Drug Dev Ind Pharm. 2015;1137306.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raval, M., Bagada, H. Formulation and Evaluation of Cyclodextrin-Based Thermosensitive In Situ Gel of Azithromycin for Periodontal Delivery. J Pharm Innov 16, 67–84 (2021). https://doi.org/10.1007/s12247-019-09422-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-019-09422-3