Abstract
Purpose
Pharmaceutically active compounds (API) in solid form have several disadvantages which may include polymorphism, poor solubility, and low bioavailability. To overcome these issues, API-based ionic liquids have been proposed to solve this problem.
Methods
Solvent evaporation method was selected to prepare ionic liquid forms of CVD. A binary mixture of CVD with citric acid, tartaric acid, and saccharin in 1:1 M ratio was dissolved in 5 ml of methanol then they left 4 days for solvent evaporation. The solubility of CVD and prepared ionic liquids were measured in different media.
Results
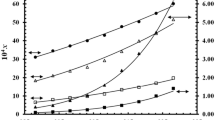
A viscous yellow liquid in all cases was obtained. More than three-unit differences between pKa of CVD and studied compounds and characterization by different instrumental analysis methods confirmed the formation of an ionic liquid form of CVD and the prepared ionic liquids could significantly change the solubility of CVD.
Conclusion
Overall, ionic liquids of CVD could be used for overcoming the disadvantages of its solid form and increasing CVD solubility. However, pH, type, and concentration of dissolution medium and the solubility of counter-ions are critical issues which they should be considered in evaluating solubility of CVD and its ionic liquid forms.







Similar content being viewed by others
References
Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65(1):315–499. https://doi.org/10.1124/pr.112.005660.
Martínez F, Jouyban A, Acree WE Jr. Pharmaceuticals solubility is still nowadays widely studied everywhere. Pharm Sci. 2017;23:1–2. https://doi.org/10.15171/PS.2017.01.
Jouyban A. Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures. J Pharm Pharm Sci. 2008;11(1):32–58.
Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59(7):645–66. https://doi.org/10.1016/j.addr.2007.05.012.
Vinarov Z, Katev V, Radeva D, Tcholakova S, Denkov ND. Micellar solubilization of poorly water-soluble drugs: effect of surfactant and solubilizate molecular structure. Drug Dev Ind Pharm. 2018;44(4):677–86. https://doi.org/10.1080/03639045.2017.1408642.
Kesisoglou F, Panmai S, Wu Y. Nanosizing—oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–44. https://doi.org/10.1016/j.addr.2007.05.003.
Keramatnia F, Shayanfar A, Jouyban A. Thermodynamic solubility profile of carbamazepine–cinnamic acid cocrystal at different pH. J Pharm Sci. 2015;104(8):2559–65. https://doi.org/10.1002/jps.24525.
Childs SL, Stahly GP, Park A. The salt-cocrystal continuum: the influence of crystal structure on ionization state. Mol Pharm. 2007;4(3):323–38. https://doi.org/10.1021/mp0601345.
Cerreia Vioglio P, Chierotti MR, Gobetto R. Pharmaceutical aspects of salt and cocrystal forms of APIs and characterization challenges. Adv Drug Deliv Rev. 2017;117:86–110. https://doi.org/10.1016/j.addr.2017.07.001.
Yadav B, Balasubramanian S, Chavan RB, Thipparaboina R, Naidu VG, Shastri NR. Hepatoprotective cocrystals and salts of riluzole: prediction, synthesis, solid state characterization and evaluation. Cryst Growth Des. 2018. https://doi.org/10.1021/acs.cgd.7b01514.
Ventura SPM, Silva FAE, Quental MV, Mondal D, Freire MG, Coutinho JAP. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: past, present, and future trends. Chem Rev. 2017;117(10):6984–7052. https://doi.org/10.1021/acs.chemrev.6b00550.
Martins MAP, Frizzo CP, Moreira DN, Zanatta N, Bonacorso HG. Ionic liquids in heterocyclic synthesis. Chem Rev. 2008;108(6):2015–50. https://doi.org/10.1021/cr078399y.
Zheng Z, Xu Q, Guo J, Qin J, Mao H, Wang B, et al. Structure-antibacterial activity relationships of imidazolium-type ionic liquid monomers, poly(ionic liquids) and poly(ionic liquid) membranes: effect of alkyl chain length and cations. ACS Appl Mater Interfaces. 2016;8(20):12684–92. https://doi.org/10.1021/acsami.6b03391.
Moniruzzaman M, Tahara Y, Tamura M, Kamiya N, Goto M. Ionic liquid-assisted transdermal delivery of sparingly soluble drugs. Chem Commun. 2010;46(9):1452–4. https://doi.org/10.1039/b907462g.
Mizuuchi H, Jaitely V, Murdan S, Florence AT. Room temperature ionic liquids and their mixtures: potential pharmaceutical solvents. Eur J Pharm Sci. 2008;33(4–5):326–31. https://doi.org/10.1016/j.ejps.2008.01.002.
Alawi MA, Hamdan II, Sallam AA, Heshmeh NA. Solubility enhancement of glibenclamide in choline-tryptophan ionic liquid: preparation, characterization and mechanism of solubilization. J Mol Liq. 2015;212:629–34. https://doi.org/10.1016/j.molliq.2015.10.006.
Faria RA, Bogel-Łukasik E. Solubilities of pharmaceutical and bioactive compounds in trihexyl(tetradecyl)phosphonium chloride ionic liquid. Fluid Phase Equilib. 2015;397:18–25. https://doi.org/10.1016/j.fluid.2015.03.053.
Shamshina JL, Kelley SP, Gurau G, Rogers RD. Develop ionic liquid drugs. Nature. 2015;528(7581):188–9. https://doi.org/10.1038/528188a.
Shamshina JL, Barber PS, Rogers RD. Ionic liquids in drug delivery. Expert Opin Drug Deliv. 2013;10(10):1367–81. https://doi.org/10.1517/17425247.2013.808185.
Miwa Y, Hamamoto H, Ishida T. Lidocaine self-sacrificially improves the skin permeation of the acidic and poorly water-soluble drug etodolac via its transformation into an ionic liquid. Eur J Pharm Biopharm. 2016;102:92–100. https://doi.org/10.1016/j.ejpb.2016.03.003.
Shadid M, Gurau G, Shamshina JL, Chuang BC, Hailu S, Guan E, et al. Sulfasalazine in ionic liquid form with improved solubility and exposure. Med Chem Commun. 2015;6(10):1837–41. https://doi.org/10.1039/c5md00290g.
Egorova KS, Gordeev EG, Ananikov VP. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev. 2017;117(10):7132–89. https://doi.org/10.1021/acs.chemrev.6b00562.
Stoimenovski J, Dean PM, Izgorodina EI, MacFarlane DR. Protic pharmaceutical ionic liquids and solids: aspects of protonics. Faraday Discuss. 2012;154:335–52. https://doi.org/10.1039/c1fd00071c.
Stoimenovski J, MacFarlane DR, Bica K, Rogers RD. Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: a position paper. Pharm Res. 2010;27(4):521–6. https://doi.org/10.1007/s11095-009-0030-0.
Hamed R, Awadallah A, Sunoqrot S, Tarawneh O, Nazzal S, AlBaraghthi T, et al. pH-dependent solubility and dissolution behavior of carvedilol—case example of a weakly basic BCS class II drug. AAPS PharmSciTech. 2016;17(2):418–26. https://doi.org/10.1208/s12249-015-0365-2.
Rasool MF, Khalil F, Läer S. Optimizing the clinical use of carvedilol in liver cirrhosis using a physiologically based pharmacokinetic modeling approach. Eur J Drug Metab Pharmacokinet. 2017;42(3):383–96. https://doi.org/10.1007/s13318-016-0353-2.
Beattie K, Phadke G, Novakovic J. Carvedilol. Profiles of drug substances, excipients and related methodology. Academic, Waltham. 2013;38:113–57.
Morgan T. Clinical pharmacokinetics and pharmacodynamics of carvedilol. Clin Pharmacokinet. 1994;26(5):335–46.
Liu D, Pan H, He F, Wang X, Li J, Yang X, et al. Effect of particle size on oral absorption of carvedilol nanosuspensions: in vitro and in vivo evaluation. Int J Nanomedicine. 2015;10:6425.
Hiendrawan S, Widjojokusumo E, Veriansyah B, Tjandrawinata RR. Pharmaceutical salts of carvedilol: polymorphism and physicochemical properties. AAPS PharmSciTech. 2017;18(4):1417–25. https://doi.org/10.1208/s12249-016-0616-x.
Loftsson T, Vogensen SB, Desbos C, Jansook P. Carvedilol: solubilization and cyclodextrin complexation: a technical note. AAPS PharmSciTech. 2008;9(2):425–30. https://doi.org/10.1208/s12249-008-9055-7.
Krstić M, Radojević M, Stojanović D, Radojević V, Uskoković P, Ibrić S. Formulation and characterization of nanofibers and films with carvedilol prepared by electrospinning and solution casting method. Eur J Pharm Sci. 2017;101:160–6. https://doi.org/10.1016/j.ejps.2017.02.006.
Wegmann M, Parola L, Bertera FM, Taira CA, Cagel M, Buontempo F, et al. Novel carvedilol paediatric nanomicelle formulation: in-vitro characterization and in-vivo evaluation. J Pharm Pharmacol. 2017;69(5):544–53. https://doi.org/10.1111/jphp.12605.
Yuvaraja K, Khanam J. Enhancement of carvedilol solubility by solid dispersion technique using cyclodextrins, water soluble polymers and hydroxyl acid. J Pharm Biomed Anal. 2014;96:10–20. https://doi.org/10.1016/j.jpba.2014.03.019.
Shayanfar A, Jouyban A. Drug-drug coamorphous systems: characterization and physicochemical properties of coamorphous atorvastatin with carvedilol and glibenclamide. J Pharm Innov. 2013;8(4):218–28. https://doi.org/10.1007/s12247-013-9162-1.
Pokharkar VB, Mandpe LP, Padamwar MN, Ambike AA, Mahadik KR, Paradkar A. Development, characterization and stabilization of amorphous form of a low Tg drug. Powder Technol. 2006;167(1):20–5. https://doi.org/10.1016/j.powtec.2006.05.012.
www.accessdata.fda.gov/scripts/fdcc/?set=SCOGS. Accessed 10 Sept 2017
www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm. Accessed 10 Sept 2017
Keramatnia F, Jouyban A, Valizadeh H, Delazar A, Shayanfar A. Ketoconazole ionic liquids with citric and tartaric acid: synthesis, characterization and solubility study. Fluid Phase Equilib. 2016;425:108–13. https://doi.org/10.1016/j.fluid.2016.05.016.
Balk A, Holzgrabe U, Meinel L. Pro et contra’ ionic liquid drugs - challenges and opportunities for pharmaceutical translation. Eur J Pharm Biopharm. 2015;94:291–304. https://doi.org/10.1016/j.ejpb.2015.05.027.
Cojocaru OA, Bica K, Gurau G, Narita A, McCrary PD, Shamshina JL, et al. Prodrug ionic liquids: functionalizing neutral active pharmaceutical ingredients to take advantage of the ionic liquid form. Med Chem Commun. 2013;4(3):559–63. https://doi.org/10.1039/c3md20359j.
Cojocaru OA, Kelley SP, Gurau G, Rogers RD. Procainium acetate versus procainium acetate dihydrate: irreversible crystallization of a room-temperature active pharmaceutical-ingredient ionic liquid upon hydration. Cryst Growth Des. 2013;13(8):3290–3. https://doi.org/10.1021/cg400686e.
Shamshina JL, Cojocaru OA, Kelley SP, Bica K, Wallace SP, Gurau G, et al. Acyclovir as an ionic liquid cation or anion can improve aqueous solubility. ACS Omega. 2017;2(7):3483–93.
Ferraz R, Branco LC, Marrucho IM, Araújo JMM, Rebelo LPN, Da Ponte MN, et al. Development of novel ionic liquids based on ampicillin. Med Chem Commun. 2012;3(4):494–7. https://doi.org/10.1039/c2md00269h.
Peng C, Chan MN, Chan CK. The hygroscopic properties of dicarboxylic and multifunctional acids: measurements and UNIFAC predictions. Environ Sci Technol. 2001;35(22):4495–501. https://doi.org/10.1021/es0107531.
Wouters J, Quéré L. Pharmaceutical salts and co-crystals. London: Royal Society of Chemistry; 2012.
Banerjee R, Bhatt PM, Ravindra NV, Desiraju GR. Saccharin salts of active pharmaceutical ingredients, their crystal structures, and increased water solubilities. Cryst Growth Des. 2005;5(6):2299–309. https://doi.org/10.1021/cg050125l.
ACD/labs 6.00 Advanced Chemistry Development
United States Pharmacopeia 30-NF25. Rockville: United States Pharmacopeial Convention; 2007
www.accessdata.fda.gov/scripts/cder/dissolution/dsp_getallData.cfm. Accessed 10 Sept 2017
Committee JPE. The Japanese pharmacopoeia. Tokyo: Hirokawa Press; 2006.
Yalkowsky SH, He Y, Jain P. Handbook of aqueous solubility data. Boca Raton: CRC press; 2010.
David SE, Timmins P, Conway BR. Impact of the counterion on the solubility and physicochemical properties of salts of carboxylic acid drugs. Drug Dev Ind Pharm. 2012;38(1):93–103. https://doi.org/10.3109/03639045.2011.592530.
Chrzanowski FA, Ahmad K. The preparation and evaluation of salt forms of linogliride with reduced solubilities as candidates for extended release. Drug Dev Ind Pharm. 2017;43(3):421–31. https://doi.org/10.1080/03639045.2016.1257019.
Acknowledgments
This article is a part of the results of S.S’s Pharm.D thesis No. 3965 registered at Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran. The authors would like to give special thanks to East Azarbaijan Sciences and Technology Park for providing DSC thermograms. We appreciate the editor and reviewers of this manuscript for editing and their valuable comments.
Funding
A.S. thanks the Ministry of Health and Medical Education (grant for young assistant professors), Tehran, Iran, for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 3997 kb)
Rights and permissions
About this article
Cite this article
Shayanfar, S., Shayanfar, A. Ionic Liquid Forms of Carvedilol: Preparation, Characterization, and Solubility Studies. J Pharm Innov 14, 382–390 (2019). https://doi.org/10.1007/s12247-018-9361-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9361-x