Abstract
Purpose
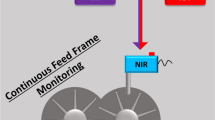
This study describes the validation of a near infrared spectroscopic method for monitoring a continuous manufacturing system. The achievement of this goal requires determining the near infrared (NIR) method’s accuracy, precision, obtaining an estimate of the sample volume analyzed, and the frequency with which measurements should be obtained.
Methods
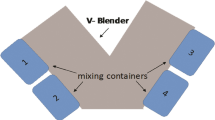
Five calibration blends were prepared spanning a concentration range from 7 to 13 % w/w ibuprofen to prepare a partial least squares (PLS) calibration model for a five component formulation. NIR spectra were obtained after powders passed through feeders and a custom-made tumble mixer. The calibration model’s precision and accuracy were determined with the prediction of three validation blends.
Results
NIR concentration predictions obtained from spectra collected during the validation runs showed relative standard errors of predictions of 2.8 % at target concentration with standard deviations NMT 0.2 % w/w. At certain time points, blend samples coming out of the blender were collected for UV analysis. Results from the NIR and UV analysis were comparable with the largest difference being 0.36 % w/w between the methods. A continuous blending process was monitored for 3 min after steady-state conditions were achieved. All individual predictions were within 3 standard deviations of the average reference value. The standard deviation for the NIR concentration predictions was 0.49 % w/w.
Conclusion
The use of the standard normal variate (SNV) transform significantly reduced the effect of differences in powder flow on the NIR predictions. The use of variograms provided valuable insight into the frequency of measurements needed for the continuous manufacturing system. Additional research is needed to investigate the differences observed in the NIR and UV results for the continuous manufacturing run.








Similar content being viewed by others
References
Bell TA. Challenges in the scale-up of particulate processes—an industrial perspective. Powder Technol. 2005;150(2):60–71.
Leuenberger H. New trends in the production of pharmaceutical granules: the classical batch concept and the problem of scale-up. Eur J Pharm Biopharm. 2001;52(3):279–88.
Mort PR. Scale-up of binder agglomeration processes. Powder Technol. 2005;150(2):86–103.
Rodriguez-Justo O, Morales ÂM. Analysis of process parameters on the characteristics of liposomes prepared by ethanol injection with a view to process scale-up: effect of temperature and batch volume. Chem Eng Res Des. 2011;89(6):785–92.
Florian M, Velázquez C, Méndez R. New continuous tumble mixer characterization. Powder Technol. 2014;256:188–95.
Jamrogiewicz M et al. Determination of API content in a pilot-scale blending by near-infrared spectroscopy as a first step method to process line implementation. Acta Pol Pharm. 2013;70(3):419–29.
Shi Z et al. Process characterization of powder blending by near-infrared spectroscopy: blend end-points and beyond. J Pharm Biomed Anal. 2008;47(4–5):738–45.
Liew CV, Karande AD, Heng PW. In-line quantification of drug and excipients in cohesive powder blends by near infrared spectroscopy. Int J Pharm. 2010;386(1–2):138–48.
Sekulic SS et al. On-line monitoring of powder blend homogeneity by near-infrared spectroscopy. Anal Chem. 1996;68(3):509–13.
Momose W et al. Process analytical technology applied for end-point detection of pharmaceutical blending by combining two calibration-free methods: simultaneously monitoring specific near-infrared peak intensity and moving block standard deviation. Powder Technol. 2011;210(2):122–31.
Vanarase AU et al. Real-time monitoring of drug concentration in a continuous powder mixing process using NIR spectroscopy. Chem Eng Sci. 2010;65(21):5728–33.
Martínez L et al. Use of near-infrared spectroscopy to quantify drug content on a continuous blending process: influence of mass flow and rotation speed variations. Eur J Pharm Biopharm. 2013;84(3):606–15.
Beach L et al. Near-infrared spectroscopy for the in-line characterization of powder voiding part II: quantification of enhanced flow properties of surface modified active pharmaceutical ingredients. J Pharm Innov. 2010;5(1–2):1–13.
Barnes RJ, Dhanoa MS, Lister SJ. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl Spectrosc. 1989;43(5):772–7.
Rinnan Å, Berg Fvd, Engelsen SB. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal Chem. 2009;28(10).
Popo M et al. Blend uniformity analysis using stream sampling and near infrared spectroscopy. AAPS PharmSciTech. 2002;3(3):E24.
Andersson M et al. NIR spectroscopy on moving solids using a scanning grating spectrometer—impact on multivariate process analysis. Chemom Intell Lab Syst. 2005;75(1):1–11.
Sulub Y et al. Real-time on-line blend uniformity monitoring using near-infrared reflectance spectrometry: a noninvasive off-line calibration approach. J Pharm Biomed Anal. 2009;49(1):48–54.
Koller DM et al. Continuous quantitative monitoring of powder mixing dynamics by near-infrared spectroscopy. Powder Technol. 2011;205(1–3):87–96.
El-Hagrasy AS et al. Near-infrared spectroscopy and imaging for the monitoring of powder blend homogeneity. J Pharm Sci. 2001;90(9):1298–307.
Berthiaux H, Marikh K, Gatumel C. Continuous mixing of powder mixtures with pharmaceutical process constraints. Chem Eng Process Process Intensif. 2008;47(12):2315–22.
Bakeev KA. Process analytical technology: spectroscopic tools and implementation strategies for the chemical and pharmaceutical industries. Wiley; 2005.
Petersen L, Esbensen KH. Representative process sampling for reliable data analysis—a tutorial. J Chemom. 2005;19(11–12):625–47.
Engisch WE, Muzzio FJ. Method for characterization of loss-in-weight feeder equipment. Powder Technol. 2012;228:395–403.
Acknowledgments
This study was financially supported by the NSF Engineering Research Center for Structured Organic Particulate Systems (EEC-0540855). The authors would like to thank Jesus Torres for assistance with the Fast Fourier Transform analysis that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colón, Y.M., Florian, M.A., Acevedo, D. et al. Near Infrared Method Development for a Continuous Manufacturing Blending Process. J Pharm Innov 9, 291–301 (2014). https://doi.org/10.1007/s12247-014-9194-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-014-9194-1