Abstract
Food availability is a key determinant of the nursery value of a given habitat for larval and juvenile fishes. Growth, survival, and recruitment success are often inter-correlated and influenced by prey availability and associated feeding success. This is likely true for the threatened population of Longfin Smelt (Spirinchus thaleichthys) in the San Francisco Estuary (SFE) which has collapsed in recent decades along with its preferred prey. In years with high precipitation and freshwater outflow, larval Longfin Smelt are found in shallow wetland habitats throughout the SFE, but variation in the availability of food and feeding success in these habitats remains unexplored. To examine spatial variation in the trophic value of different rearing habitats, we quantified variation in prey availability, feeding success, and prey selection for larval and juvenile Longfin Smelt captured in restored tidal marshes, sloughs, and open-water habitats in the northern and southern SFE. Prey abundance varied spatially, with densities approximately tenfold greater in southern sloughs and restored tidal ponds relative to northern and open-water habitats. Feeding success of larval Longfin Smelt was positively correlated with both fish length and prey density. Larval Longfin Smelt fed selectively on the copepod Eurytemora affinis, with larger individuals (> 25 mm total length) exhibiting an ontogenetic diet shift to larger mysid shrimps. Our results suggest that wetland habitats across the SFE vary greatly in their trophic value, with previously unexplored habitats exhibiting the highest densities of prey and the highest foraging success for larval Longfin Smelt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuaries provide vital nursery habitats for many migrant and resident fish species (Boehlert and Mundy 1988; Sheaves et al. 2014; Lefcheck et al. 2019). High concentration of suitable larval prey is one factor of estuaries that can improve feeding success and promote the growth and survival of fishes through their vulnerable larval life stage (Houde 1978, 1987, Lusardi et al. 2019). Zooplankton production in estuarine habitats is often stimulated by the delivery of particulate organic matter and nutrients via both runoff from upstream watersheds and tidal transport from downstream coastal habitats (Odum 1961; Schelske and Odum 1962; Malone et al. 1988). Larval fish survival can also be enhanced through the high turbidity often found in estuaries which can provide increased protection from predators (Moore and Moore 1976; Rypel et al. 2007). Thus, the abundance and quality of estuarine habitats can greatly influence the population dynamics of coastal fishes.
However, not all estuarine habitats contribute equally to adult populations and a variety of factors can influence variability in the overall recruitment of individuals. The amount of successful recruitment proportional to the size of the available habitat can help determine the relative value of the “nursery habitat” (Beck et al. 2001). Nursery value can be determined by examining several interacting factors that influence the survival of recruits (Beck et al. 2001). These factors include biotic processes (larval supply, predation, food availability), abiotic processes (hydrology, physio-chemical properties, tides), and landscape features (connectivity, area, fragmentation) (Sheaves et al. 2014). Survival of young fish is often driven by direct mortality due to predation (Houde 1987; Anderson 1988; Bergenius et al. 2002), and these mortality rates generally decrease as the fish grow, resulting in a positive relationship between growth rate and survival (Houde 1987; Anderson 1988; Bergenius et al. 2002) (Fig. 1). Growth rate is largely influenced by prey concentrations and feeding success (Houde 1978, 1987), and feeding success can further increase growth rate leading to even greater feeding success, growth, and survival (China and Holzman 2014; Levy et al. 2017). Prey availability is a key feature of a habitat that strongly influences feeding success and the habitats nursery value for a given fish species.
Conceptual model detailing how feeding success and prey availability (outlined in red) can help determine the relative quality of potential nursery habitat. Numbered citations are as follows: 1. (Beck et al. 2001) 2. (Houde 1987; Bergenius et al. 2002; Anderson 1988) 3. (Houde 1978, 1987) 4. (Levy et al. 2017, China et al. 2014) 5. (Dill et al. 1984). (Color figure online)
Estuaries are threatened on multiple fronts: from land development (Lotze et al. 2006), harmful algal blooms (Burkholder et al. 2002), freshwater diversions (Nichols 1986), and rising sea levels (Scavia et al. 2002). Consequently, estuaries have been largely degraded across much of the world (Barbier et al. 2011), including the San Francisco Estuary (SFE), one of the largest estuaries in North America. Approximately 95% of the tidal marsh habitat of the SFE was diked and filled in during the twentieth century (Nichols et al. 1986), and in some years, a large proportion of river flows are diverted for urban and agriculture use (Hutton et al. 2017a, b). Conservation efforts have been initiated to improve the quality of wetland habitats in the SFE over the last several decades, with notable restoration projects within the upper and lower regions of the estuary (Macvean et al. 2011; Williams and Orr 2002; Valoppi 2018).
Though highly degraded, the SFE remains home to the genetically distinct, southernmost population of Longfin Smelt (Spirinchus thaleichthys), a pelagic forage fish in the family Osmeridae that is native to coastal and estuarine habitats of the northeastern Pacific Ocean (Garwood 2017; Sağlam et al. 2021). The abundance of SFE Longfin Smelt has declined since the 1970s, prompting their listing as threatened under the California Endangered Species Act in 2009 (Moyle 2002; CDFG 2009). Longfin Smelt exhibit a semi-anadromous life history, moving upstream to low-salinity tidal habitats during California’s cool, wet winters to spawn (Moyle 2002). Larval (< 34 mm TL; Wang 1986) life stages rear in both open water and tidal wetland habitats through the early spring, after which they migrate seaward into deeper, cooler bay and coastal marine habitats during warmer summer months (Moyle 2002; Rosenfield and Baxter 2007; Garwood 2017).
Longfin Smelt are pelagic zooplanktivores, with larvae consuming copepods and juveniles and adults consuming larger crustaceans, especially mysid shrimp (Chigbu and Sibley 1994, 1998; Feyrer et al. 2003; Hobbs et al. 2006). In the upper SFE, larval Longfin Smelt primarily consume copepods including Pseudodiaptomus forbesi and Acanthocyclops vernalis (Hobbs et al. 2006), while adults likely prey upon several mysid species; however, details regarding this ontogenetic dietary shifts remain unexplored. Both P. forbesi. and A. vernalis are non-native copepods introduced in the late 1900s and have since become the most abundant calanoid copepods in the upper estuary, overtaking Eurytemora affinis, which was previously the dominant prey item for many planktivorous fishes in the upper SFE (Winder and Jassby 2011). Changes in the community structure of zooplankton have corresponded with regional declines in total zooplankton biomass and pelagic fishes in the upper estuary (Feyrer et al. 2003; Kimmerer 2006; Sommer et al. 2007). These declines were likely caused by a combination of factors including decreased freshwater outflows and overgrazing from the invasive overbite clam (Potamocorbula amurensis) (Kimmerer 2002; Cloern and Jassby 2012; Hammock et al. 2019).
The early life stages of Longfin Smelt utilize tidal wetlands throughout the SFE, and their distributions are driven largely by variation in freshwater outflow (Dege and Brown 2004). In dry years, larvae are often found in the upper estuary, including Suisun Bay and the Delta (Grimaldo et al. 2017), but appear to be dispersed further downstream in tidal wetlands and open water habitats of the northern and southern SFE during years of high precipitation and freshwater outflow (Lewis et al. 2020; Grimaldo et al. 2020). In years with above normal precipitation, when fish are widely dispersed across the estuary, Longfin Smelt recruitment is maximized (Kimmerer 2002; Rosenfield and Baxter 2007; Kimmerer et al. 2009), suggesting that rearing habitats may vary in their relative contributions to recruitment. However, it remains unknown how prey availability varies among regions and habitat types of the San Francisco Estuary and how such variation influences the feeding success of Longfin Smelt.
In 2017, a year of high precipitation and warm waters, early life stage Longfin Smelt (and their prey) were observed and collected throughout the northern and southern San Francisco Estuary, and for the first time in Alviso Marsh in the South Bay (Lewis et al. 2020). These collections provided an opportunity to examine spatial variation in feeding selectivity and success across regions, habitats, and in relation to prey availability. We quantified spatial variation in prey density and feeding success in 2017 for Longfin Smelt to assess regional and habitat-type differences in two wetlands of the SFE. Feeding success was examined as a function of fish size and prey density to assess the relative influence of each factor, and prey selectivity was examined by contrasting the composition of prey in fish stomachs with that of the ambient environment at capture. Identification of the habitats that best increase food supply and feeding success of Longfin Smelt is a critical step for developing effective conservation and restoration efforts for this imperiled species.
Materials and Methods
Study Region
The SFE (Fig. 2) is the largest estuarine embayment on the west coast of the Americas. It is divided into five unique biogeographic regions. The Delta, Confluence, and linked Suisun regions form the “upper estuary” which receives the majority of freshwater flows into the system. Carquinez Strait separates the upper estuary from the more brackish habitats of San Pablo Bay and its tributaries, collectively referred to as the “northern SFE.” The “southern SFE” consists of South San Francisco Bay and the lagoonal Lower South San Francisco Bay (LSB), two shallow embayments which receive relatively little freshwater flows compared to the northern SFE. The northern and southern SFE are separated by the large expanse of the Central Bay, which connects to the Pacific Ocean and is surrounded by a mostly urbanized landscape. Seasonality of the SFE’s precipitation drives salinity conditions throughout the estuary and its tidal marshes, with fresh to low salinity conditions prevailing in the wet winter and spring conditions and higher salinities occurring in the drier summer and fall. Seasonal control of salinity in the estuary can also be highly variable, as the influence of climate change increases the frequency and magnitude of droughts and floods in the region (Mann and Gleick 2015).
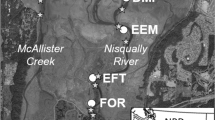
(a) Zooplankton and Longfin Smelt samples were collected across the upper (Suisun, Confluence, North and South-East Delta), northern (San Pablo Bay), and southern (Lower South Bay) regions of the SFE, including the associated tributaries and marsh complexes. California Department of Fish and Wildlife (CDFW) Environmental Monitoring Program (EMP) zooplankton sampling is also shown. Habitat types and sampling locations are shown in more detail for the northern SFE (b) and southern SFE (c)
Both the northern and southern SFE are heavily altered landscapes, with large sections of historical tidal marsh having been converted into industrial salt ponds during the twentieth century, or developed for agriculture and urbanization (Nichols et al. 1986; Whipple et al. 2012). Efforts to restore many of these degraded wetland habitats are on-going, including restoration of 6000 acres of solar-evaporation salt ponds in the Napa-Sonoma Marsh Complex (in the northern SFE) and the Alviso Marsh Complex (in the southern SFE), where active restoration of tidal action to the previously isolated salt ponds has been implemented over the last two decades (Williams and Orr 2002; Valoppi 2018). The restored ponds are connected to adjacent subtidal sloughs that move water, nutrients, sediments, and organisms to and from restored pond habitats with the tidal current. These smaller slough habitats are connected to larger open expanses of water in San Pablo Bay in the north and Lower South San Francisco Bay in the south. The unique characteristics of open bay, slough, and restored pond habitats likely influence the food availability and feeding success of many species of estuarine fishes.
Field Collection
Zooplankton samples were collected using a modified Clarke-Bumpus (CB) net (approximately 12-cm diameter opening) with a 160-µm mesh attached to a 20-mm net frame (described below), allowing us to contrast prey availability with fish catch and diet data. Zooplankton samples were collected in March–April of 2017 throughout each marsh complex (Napa-Sonoma, Petaluma, and Alviso) as well as San Pablo Bay and the Lower South San Francisco Bay (Fig. 2). To compare diets and prey availability for fish captured in otter trawls, a CB net was attached to a D-frame mysid net and towed in an oblique step-wise fashion directly prior to or after otter trawl sampling. This zooplankton sampling method was comparable to zooplankton samples collected with the CB net attached to the 20-mm net. Estimates of the water volume sampled by each CB zooplankton tow were calculated (Eq. 3) using flowmeter revolutions (General Oceanics Flowmeter 2030R, Miami FL, USA) for each tow and a standard constant (k = 0.0269). The flowmeter was attached to the center of the opening of the CB net. Zooplankton samples were stored in a 10% formalin solution for later species identification and quantification.
Longfin Smelt were collected in March–April of 2017 across regions (northern and southern SFE (Fig. 2a) and habitat types (restored pond, slough, and open bay) (Fig. 2b, c) from surveys conducted in March and April of 2017 (Table 1). Due to difficulty accessing restored ponds in the northern SFE, sampling was insufficient and no Longfin Smelt larvae were collected in restored ponds in those areas. Only sloughs and open bay habitats were included in regional comparisons and only samples from the southern SFE were used to contrast the three habitat types.
Longfin Smelt were collected using an ichthyoplankton net (California Department of Fish and Wildlife 20 mm Survey net) and otter trawl (mimicking the Suisun Marsh Study otter trawl). The 20-mm net (named 20 mm because it was designed to retain fish ~ 20 mm in total length) is a 1600-µm-mesh plankton net mounted to a D-shaped frame with skids (5.1-m length, 1.5-m2 net mouth opening). Surveys using the 20-mm net were conducted bi-weekly at each sampling site from March to April to encompass the period in which Longfin Smelt are present in the region. Sampling sites in the northern SFE were determined randomly across the region, while in the southern SFE, fixed sites were selected to achieve spatial dispersion throughout the South Bay (Lewis et al. 2019). Otter trawl surveys (1.5 m × 4.3 m opening, 35-mm stretch mesh body, 6-mm stretch liner) were conducted monthly in each region to monitor juvenile and adult fishes, but also captured larval Longfin Smelt (> 18-mm fork length) in March and April. Otter trawls were pulled along the bottom for 10 min into the current, while the 20-mm net was pulled in a step-wise oblique fashion for 10 min into the current. Longfin Smelt collected in the field were euthanized and stored in 95% ethanol for later diet and otolith processing.
The spring of 2017 was a period of high precipitation and runoff, with high flows across SFE tributaries. During the sampling period, salinities were similar in the northern and southern SFE, where values were higher in the open bay habitats than in the restored ponds or sloughs. Dissolved oxygen was lower in the southern SFE habitats, while temperatures there tended to be higher (Fig. S1). All samples were collected during daylight between 7 a.m. and 1 p.m.
Laboratory Methods
Zooplankton samples (N = 30) collected with 20-mm net samples containing Longfin Smelt were selected to cover the same factors of region and habitat type (Table 1). Longfin Smelt captured in the otter trawls, which could not have an attached CB sample, were paired with CB samples collected directly prior to or after the otter trawl. Samples were initially placed in a 50-µm sieve and rinsed with water to reduce formalin fumes. The sample was then placed in a beaker, gently homogenized, and diluted until a 1-mL subsample drawn with a pipette yielded between 200 and 400 organisms. Dilution volume for samples ranged between 25 mL and 2.5 L. Once the desired concentration was achieved, a 1-mL subsample was pipetted out and placed on a Sedgewick Rafter counting cell. All organisms in the subsample were then identified under a compound microscope to the lowest possible taxon and quantified. Three 1-mL subsamples were processed for each sample. The counts of each taxon in each subsample were then multiplied by the sample dilution volume to estimate the total number of individuals of that taxon in the full sample (Eq. 1).
Where Niu is the estimated total count of taxon i in the sample calculated from subsample u, D is the dilution volume used for sample, and niu is the count of taxon i in the 1-mL subsample u. For each sample j, the 3 estimated taxon counts (Niu) were then averaged to provide a single total count (Nij) of each taxon i in each sample. Because mysids were too large to be consistently sampled by the pipette, Nij for mysids was counted directly, with all mysids in each sample removed and counted directly without subsampling. Most mysids sampled in the CB net had not yet developed diagnostic characteristics and were classified as unidentified mysids.
A total of 178 individual Longfin Smelt were selected for diet analyses, with samples stratified among size classes, habitats, and regions (Table 1). No Longfin Smelt were captured in restored ponds in the northern SFE during the study period, so comparisons of feeding success in tidal restored habitats were restricted to southern habitats. Fish were photographed and then measured for total length (TL mm) using Adobe photoshop software (Photoshop CC 2017, Adobe, San Jose). For diet analysis, a dissecting scope, tweezers, and scalpel were used to remove and open the stomach and gut tract of each fish. Feeding incidence was recorded as positive (1) when any food items were present in the stomach and/or gut tract and absent (0) when no prey items were present. Stomach and gut contents were placed in a drop of water on a Sedgewick Rafter counting cell and identified to the lowest possible taxon and measured to nearest 0.1 mm under a compound microscope.
The total wet biomass (μg) of prey in either fish diets or zooplankton samples (j) was calculated by multiplying the count of each prey type by the corresponding mean taxon-specific biomass (for mysids, biomass was calculated using a length-mass curve). The estimated masses of all prey taxa were then summed for each fish or zooplankton sample (Table S1) (Kimmerer 2006; Slater and Baxter 2014) (Eq. 2).
Where Pj is the biomass of all prey taxa in fish or sample j, Nij is the count of prey taxa i in fish or sample j, and Bi is the individual average biomass of prey taxa i (or calculated biomass if mysid) (Table S1). These calculations yielded estimates of total counts and biomass of each prey type in each stomach or zooplankton sample. Since biomass was calculated using estimates of numerical density and static estimates of the biomass for each taxon, calculations using mean mass estimates did not account for within-group variation in size. Prey counts for each sample were multiplied by the taxon-specific biomass to yield prey-specific biomass estimates for each sample (Pij), and then divided by the sample volume (Vj) to yield biomass density estimates (Dij) of prey i (mg m−3) for sample j (Eq. 3).
The volume of water (m3) sampled for each collection (Vj) was calculated using the change in flow meter values between the beginning and end of each tow (Eq. 4).
Where Vj is the volume of sample j, dfj is the difference in beginning and ending flowmeter readings, k is a standard flowmeter constant (k = 0.0269, General Oceanics) confirmed by calibration, and a is the area of the mouth of the net (0.0113 m2).
Statistical Analyses
The biomass of available zooplankton prey was modeled as a function of region, habitat, and their interaction so that log10(Dij) ∼ f (region + habitat + region * habitat). Zooplankton densities in the northern and southern SFE were also compared qualitatively with those measured in the upper estuary during the same period (spring 2017) by the CDFW Environmental Monitoring Program, which uses similar sampling methods (EMP, https://wildlife.ca.gov/Conservation/Delta/Zooplankton-Study) (Hennessey 2018). This was done to compare prey densities between potential nursery habitats sampled in this research, and other potential Longfin Smelt nursery habitats in the SFE.
To quantify variation in the taxonomic composition of zooplankton communities among regions and habitats, the eleven most common zooplankton taxa, representing more than 93% of zooplankton collected from CB sampling, were selected. Densities of each common taxa were fourth-root transformed and used to generate a Bray–Curtis dissimilarity matrix contrasting the pairwise differences in community structure between samples (Minchin 1987). Permutational multivariate analysis of variance (PERMANOVA) was conducted using the vegan R package (Oksanen et al. 2019) to test whether differences in community structure among regions and habitats were significant (Prentice 1977; Anderson et al. 2006). All analyses were conducted using the R programming software (R version 4.0.2 Core Team 2018).
Feeding success for each fish was defined by the feeding incidence, a binary metric based on the presence (incidence = 1) or absence (incidence = 0) of prey in the gut. Logistic regression models were used to examine the effects of available zooplankton biomass and individual fish length on feeding success across habitats, regions, and their interactions (each as fixed factors).
For each fish, selectivity for each prey item was measured using Manly’s Alpha index (α) which was calculated using a subset of paired fish-diet and zooplankton samples. Manly’s Alpha index compares the relative frequency of each prey taxon in a fish’s gut to its relative frequency in the total available prey in the environment, frequency being based on abundance (Chesson 1983) (Eq. 5) (Table S2).
Where αi is the selectivity index for taxa i, ki is the proportion of prey taxa i in the diet, pi is the proportion of prey taxa i available in the environment, and m is the number of prey types available (m = 10) found across all larvae diets examined. Since 1/m (0.10) represents neutral selection for prey type i, then αi > 0.10 represents positive selection and αi< 0.10 represents negative selection (Chesson 1983).
Larger individuals appeared to have stomach contents dominated by mysid shrimp (see “Results”), so a logistic regression of mysid incidence vs. total length was developed to estimate the size at which Longfin Smelt began feeding on mysids. For example, Longfin Smelt < 25 mm TL appeared copepod-dependent, whereas those > 25 mm TL had begun to forage on mysid shrimp. Using this threshold for the ontogenetic diet shift, separate models of prey selectivity and feeding success were constructed for each of these two size classes.
Few Longfin Smelt larvae were observed in restored ponds in the northern SFE; thus, only sloughs and open-bay habitats were included in regional comparisons and only samples from the southern SFE were used to contrast all three habitat types. Models were selected by comparing the variance explained (r2) and corrected Akaike information criterion (AICc) among a set of nested models (Tables S4–S8) using the MuMIn R package (v. 1.43.6). Model assumptions were checked by examining residuals and Q-Q plots; when necessary, data was log-transformed to meet model assumptions.
Results
Zooplankton Biomass
The biomass density of available zooplankton prey ranged from 76 to 1781 mg m−3 (Fig. 3a). Nearly 40% of the variation in zooplankton biomass among samples was explained by region (northern or southern SFE), habitat type (slough or open bay), and their interaction (Table 2). In the southern SFE alone, 43% of the variation in available zooplankton biomass was explained by the habitat type when including restored ponds. While the sample size was small and variability was high, samples in southern sloughs exhibited on average 10 times higher biomass than northern sloughs (Fig. 3a; Table 2) (t(23) = 2.62, GLM, p = 0.061). While zooplankton biomass in the northern SFE was comparable to those regions sampled by the CDFW study, biomass found in the southern SFE sloughs and restored ponds of the Alviso Marsh complex was tenfold greater than most habitats in the upper SFE, except for Suisun Marsh where biomass was similar to those observed in southern restored ponds (Fig. 3a).
a Biomass of zooplankton among regions and habitats of the San Francisco Estuary (March–April 2017). Grey boxes (right panel) indicate data from regions of the eastern SFE provided by the CDFW Environmental Monitoring Program (EMP) during the same sampling period. Sample sizes in parentheses. (http://www.dfg.ca.gov/delta/projects.asp?ProjectID=ZOOPLANKTON). b Proportion of CB zooplankton biomass for each region, by taxa. For summary data, model selection, and model results, see Tables S3 and S4. (Color figure online)
a Feeding incidence correlation with Longfin Smelt total length (mm) and b feeding incidence correlated with available prey biomass (mg). Additive model used for I ~ f(l + bm, family = logistic) where I = feeding incidence, l = total length (mm), and bm = available biomass (mg). N = 178. For model results see Table 4
Zooplankton Composition
Community compositions indicated varied zooplankton prey communities in the northern versus southern SFE (PERMANOVA Bray–Curtis distance ~ region*habitat, p = 0.001; Table 3), and among the three habitat types in the southern SFE (p = 0.001). All sampled habitats were generally dominated by the calanoid copepod Eurytemora affinis, which accounted for more than 75% of zooplankton biomass in most parts of the northern and southern estuary (Fig. 3b). However, this was not the case in the more saline open bay habitat in the southern SFE, where we found high concentrations of the calanoid copepods Acartia spp. and Acartiella spp. The northern SFE habitats also had higher proportions of cyclopoid copepods compared to the southern SFE habitats.
Feeding Success
Longfin Smelt ranged from 9 to 38 mm TL across the study region, and 60% had at least one prey item in their stomachs. Both total length (z = 2.39, p = 0.0348) and the available prey biomass (z = 2.55, p = 0.011) were positively correlated with feeding incidence, together explaining 10.5% of the variation (Fig. 4; Table 4). When including all sizes of Longfin Smelt, feeding incidence did not vary among regions and habitats (Table 5). Narrowing the size range to just larval Longfin Smelt ≤ 25 mm TL, limited the analysis to only Longfin Smelt that were copepod dependent, and this resulted in spatial heterogeneity in feeding incidence (p = 0.013; Table 5; Fig. 5) that corresponded largely with patterns in prey abundance. Larval Longfin Smelt captured in the sloughs of the southern SFE (where copepods were most abundant) were approximately 30% more likely to have a positive feeding incidence than those from other regions or habitats (z = 2.199, p = 0.013).
Prey Selectivity
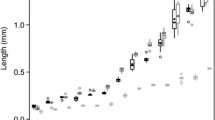
Longfin Smelt disproportionately consumed the copepod Eurytemora affinis across all habitat types and regions (Fig. 6a; Table S2). Though E. affinis was generally preferred, some Longfin Smelt also contained larger mysid shrimp in their diets (which were up to tenfold greater in individual biomass relative to the average copepod). Total length of the fish explained 46% of the variance in the occurrence of mysid shrimp in Longfin Smelt diets, with fish beginning to exhibit prey supplementation with mysids at around 26 mm TL (z = 4.66, p = 2.66e−06; Fig. 6b). Mysid shrimp accounted for over 80% of the prey mass in the stomach contents of the Longfin Smelt greater than 25 mm TL (Fig. 6c); however, these individuals still exhibited strong selection for E. affinis numerically.
a Selectivity index for 85 fish of all size classes of Longfin Smelt grouped across 25 tows, showing strong selection for copepod Eurytemora affinis. Points above dashed line are positively selected for. b Incidence of mysid prey in Longfin Smelt diets as a function of total length. Mysids appear in the diets of Longfin Smelt at approximately 25 mm in TL. Data points were jittered to more easily display relative density. Mysid art by Arthur Barros. c Proportion of the diet by mass of each prey item for Longfin Smelt ≤ 25 mm TL and d Longfin Smelt ≥ 25 mm TL. Copepod and mysid art by Arthur Barros. Larval and juvenile fish art by Adi Khen
Discussion
Assessing the nursery value of estuarine habitats is increasingly valuable for marine conservation (Beck et al. 2001; Vasconcelos et al. 2011; Sheaves et al. 2014). Due to the importance of feeding for growth and survival, the degree of spatial and temporal overlap of recruits and prey is often a key driver of recruitment strength (Durant et al. 2007) and can vary greatly with fluctuations in environmental conditions and climate (Kristiansen et al. 2011). The extent of spatial and temporal overlap of fish and their prey in the SFE is not well understood and limited to studies of Delta Smelt (Hypomesus transpacificus) and salmonids of the region (Rose et al. 2013; Herbold et al. 2018). Here, we described spatial heterogeneity in the availability of key food items for larval Longfin Smelt and demonstrated that feeding incidence is directly correlated with prey availability. Furthermore, Longfin Smelt larger than 25 mm TL exhibited increased feeding incidence and ability to catch larger, higher value mysid prey, supporting the importance of growth in determining fitness (Anderson 1988; Bergenius et al. 2002; Meekan et al. 2006). These results, in conjunction with the nursery value hypothesis, suggest that habitats throughout the SFE likely exhibit different value with respect to feeding and the growth and survival of Longfin Smelt. Previously unsampled natural and restored wetland habitats may provide some of the most valuable rearing conditions for larval Longfin Smelt, especially in years with above normal precipitation. Understanding the factors that drive this variability can greatly inform future conservation efforts for this imperiled species.
Most Longfin Smelt fed selectively on the abundant calanoid copepod Eurytemora affinis, with fish > 25 mm TL supplementing their diets with larger mysid shrimp. Though populations of both prey items have diminished significantly in the upper estuary (Feyrer et al. 2003; Winder and Jassby 2011; Cloern and Jassby 2012), they appear to remain seasonally abundant and important prey of Longfin Smelt in the northern and southern SFE. The high densities of Eurytemora affinis found in our sampling efforts were contrary to the historical trend in the upper SFE, where the taxa have been generally replaced by the calanoid copepod Pseudodiaptomus forbesi (Hennessy 2018).
Copepod biomass varied among regions and habitat types and were correlated with feeding success. The highest copepod biomass and feeding success was observed in marsh-associated habitats, especially in sloughs of the Alviso Marsh Complex in the southern SFE, where copepod biomass was on average tenfold greater than upper SFE habitats, though results were not statistically different due to high variability in the south. This variability is likely due to the physical transport of zooplankton downstream of the study area due to recent storms and pulses of tributary discharges. For example, the two low-biomass zooplankton samples in the Alviso Marsh occurred immediately following strong flushing by a recent storm. In contrast, all samples collected in southern sloughs prior to this storm exhibited much higher biomass of zooplankton than in northern habitats; removing the post-storm samples from the analysis resulted in a difference between regions (t(21) = 9.70, GLM, p = 0.041). Thus, while our results indicate large regional differences in zooplankton densities, additional sampling is needed to improve confidence in these results.
Longfin Smelt larvae exhibited positive selection for the copepod Eurytemora affinis across all regions and habitat types in the northern and southern SFE. E. affinis was historically the dominant copepod in the upper estuary, but densities declined dramatically in the 1980s (Cloern and Jassby 2012) and it has been largely replaced by another invasive calanoid copepod Pseudodiaptomus forbesi (Winder and Jassby 2011). Though Longfin Smelt in the upper estuary can feed on P. forbesi (Hobbs et al. 2006), this prey species is generally confined to low-salinity regions of the estuary (Kimmerer et al. 2018). The decline of their historically preferred prey, E. affinis, has corresponded with dramatic declines in Longfin Smelt populations in the SFE (Kimmerer 2002; Sommer et al. 2007). High densities of this important prey item in the southern SFE tributaries and marshes suggest that these regions may serve as critical habitats for Longfin Smelt, especially in years with above normal precipitation when fish and low-salinity habitats are distributed broadly throughout the SFE.
Ontogenetic prey supplementation was exhibited by Longfin Smelt when they reached 25 mm TL, with fish above this size beginning to consume larger mysid prey. This prey supplementation was occurring even before the fish reached the typical size of juveniles (≥ 34 mm TL) (Wang 1986). These larger Longfin Smelt still exhibited positive selection for E. affinis, even though the majority of the biomass in their diets was made up of much larger mysid shrimp (Fig. 6). This appears to be an important ontogenetic transition in the life history of Longfin Smelt, when larger individuals begin targeting these larger crustaceans that are the preferred prey of adult Longfin Smelt (Chigbu and Sibley 1994, 1998; Hobbs et al. 2006). Like E. affinis, the decline of mysid shrimp in the upper SFE has resulted in dietary shifts and declines for many fishes in the region, including Longfin Smelt (Kimmerer 2002; Cloern and Jassby 2012; Feyrer et al. 2003). While this trend is thoroughly documented in the upper SFE, available data on zooplankton density is sparse in the lower SFE, so historical trends cannot be compared.
Slough habitats of the Alviso Marsh Complex (AMC) exhibited the highest copepod biomass. One possible explanation for this is the high rate of nitrogen loading from the San Jose-Santa Clara Regional Wastewater Facility (SJSCRWF) (Liu et al. 2018). The SJSCRWF alone releases up to 100 million gallons per day of tertiary-treated wastewater, containing 5300 kg of nitrate, into slough habitats of the AMC (SJSCRWF 2015). This high input of nutrients in the region, along with higher water residence times (Walters et al. 1985), likely fuels higher levels of primary and secondary production in the region. For example, high chlorophyll-α concentrations (15 mg/m3) have been observed in Lower South Bay, adjacent to the AMC, in contrast to other parts of the estuary (e.g., 8 mg/m3) (Senn and Sutula 2015). These high nutrient inputs, coupled with relatively high residence times of water compared to the northern reaches of the estuary, could be stimulating primary production with high concentrations of phytoplankton supporting dense aggregations of copepods that are preyed upon by fish such as Longfin Smelt (Thebault et al. 2008; Liu et al. 2018). Similar dynamics have been proposed in Suisun Marsh, which receives treated wastewater from nearby regional wastewater treatment plants. While it has been determined that sewage inputs may play a role in regulating the availability of lower trophic food items in Suisun, it may not be as important as other factors such as turbidity, clam grazing, and freshwater flows (Cloern et al 2014). While food production and feeding success for larval Longfin Smelt were higher in the AMC during this study, accurately determining the region’s contribution to successful recruitment of Longfin Smelt in the greater SFE will require further study.
Recent investigation into the feeding of larval Longfin Smelt using DNA metabarcoding analysis suggests an added layer of diversity not captured in this and other optical based studies, where identification of zooplankton prey can be made difficult due to digestive damage (Jungbluth et al. 2021). The DNA analysis also shows that the copepods identified as Eurytemora affinis in the SFE are genetically more similar to the copepod Eurytemora carolleeae; however, here we continue to identify the species as E. affinis to remain in alignment with current CDFW taxonomic protocols.
Larger Longfin Smelt in our study exhibited increased feeding incidence, supporting the classic “bigger is better” hypothesis which suggests that larger fish have higher feeding success and corresponding growth rates that likely lead to reduced predation mortality and higher survival (Anderson 1988; Bergenius et al. 2002; Meekan et al. 2006). Higher densities of prey items in southern sloughs positively correlated with higher feeding incidence in the smaller larval fish, showing evidence of a type 1 functional response curve to prey density (Holling 1959), where feeding incidence increases with prey density up to a maximum. This could result in a positive feedback loop where higher feeding success leads to increased growth rates, and in turn, increased feeding success and growth (Fig. 1). Small fluctuations in the growth and mortality rates of larval fishes can have disproportionate effects on population dynamics (Houde 1987). Young fish that have slower growth rates spend more time in vulnerable larval stages and are more susceptible to predation and other sources of mortality (Houde 1987). Controlled laboratory studies with larval Sea Bream (Archosargus rhomboidalis), Bay Anchovy (Anchoa michilli), and Lined Sole (Achirus lineatus) showed higher rates of growth and survivorship when larval fish were stocked with higher concentrations of copepod prey (Houde 1978, 1987). Recent experiments on the growth of juvenile Coho Salmon (Oncorhynchus kisutch) in the Shasta River showed that high prey density buffered the negative effects of increased temperatures, indicating that ecosystem productivity may provide some offset from the effects of climate change (Lusardi et al. 2019).
To further explore the contributions of the under sampled regions of the SFE towards recruitment of Longfin Smelt, we can suggest several management directions. Expansion of long-term zooplankton and larval-juvenile smelt sampling efforts into multiple habitats of the northern and southern SFE would improve our understanding of trends in food abundance and Longfin Smelt recruitment in these regions. Comparisons of zooplankton densities among natural and restored wetlands across the entire estuary could help identify how wetland restoration might benefit Longfin Smelt through periods of high variability in precipitation. Quantification of nutrient and chl a concentrations across wetland habitats throughout the SFE could lead to a better understanding of how wastewater-derived nutrients might affect the trophic functioning of nursery habitats.
The spatial variation in prey density and feeding success observed in this study could have important implications for the population dynamics of Longfin Smelt. For example, higher flows in wetter years expand the volume of suitable habitat throughout the SFE and disperse more larvae from upstream regions (e.g., Suisun Bay and the Delta), where prey populations have collapsed, to downstream habitats where prey can be 10 times more abundant. This dispersal and expansion of available habitat during wet years could contribute to enhanced feeding, survival, and recruitment of Longfin Smelt. Given the large-scale declines in zooplankton and fishes in the upper SFE (Sommer et al. 2007; Baxter et al. 2008) and the positive relationships between freshwater outflow, downstream dispersal, and recruitment success of Longfin Smelt (Kimmerer 2002; Nobriga and Rosenfield 2016; Lewis et al. 2020), it is likely that habitats of the northern and southern SFE, including restored wetlands, provide valuable rearing habitats in years with above normal precipitation. By quantifying spatial patterns in prey density and feeding success of larval Longfin Smelt across the estuary, we can improve our understanding of the population dynamics and enhance the effectiveness of conservation efforts for this imperiled fish.
References
Anderson, J.T. 1988. A review of size dependant survival during pre-recruit stages of fishes in relation to recruitment. Journal of Northwest Atlantic Fishery Science 8: 55–66.
Anderson, M.J., C.J.F. Braak, and T. Braak. 2006. Permutation Tests for Multi-Factorial Analysis of Variance 73: 85–113.
Barbier, Edward B., Sally D. Hacker, Chris Kennedy, Evamaria W. Kock, A.C. Stier, and B. R. S. 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81: 169–193.
Baxter, R., F. Feyrer, M. Nobriga, and T. Sommer. 2008. Pelagic organism decline progress report: 2007 synthesis of results.
Beck, M., K. Heck, K. Able, D. Childers, D. Eggleston, and B. Gillanders. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51: 633–641.
Bergenius, M.A.J., M.G. Meekan, D.R. Robertson, and M.I. McCormick. 2002. Larval growth predicts the recruitment success of a coral reef fish. Oecologia 131: 521–525.
Boehlert, G.W., and B.C. Mundy. 1988. Roles of behavioral and physical factors in larval and juvenile fish recruitment to estuarine nursery areas. American Fisheries Society Symposium 3: 1–67.
Burkholder, J.M., P.M. Glibert, and J.M. Burkholder. 2002. Harmful algal blooms and eutrophication nutrient sources, composition, and consequences. Estuaries 25: 704–726.
CDFG. 2009. A Status Review of the Longfin Smelt (Spirinchus Thaleichthys) in California. Sacramento.
Chesson, J. 1983. The estimation and analysis of preference and its relationship to foraging models. Ecology 64: 1297–1304.
Chigbu, P., and T.H. Sibley. 1994. Diet and growth of longfin smelt and juvenile sockeye salmon in Lake Washington. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen 25 (4): 2086–2091.
Chigbu, P., and T.H. Sibley. 1998. Feeding ecology of longfin smelt (Spirinchus thaleichthys Ayres) in Lake Washington. Fisheries Research 38: 109–119.
China, V., and R. Holzman. 2014. Hydrodynamic starvation in first-feeding larval fishes. Proceedings of the National Academy of Sciences 111: 8083–8088.
Cloern, J.E., and A.D. Jassby. 2012. Drivers of change in estuarine-coastal ecosystems: Discoveries from four decades of study in San Francisco Bay. Reviews of Geophysics 50: 1–33.
Cloern, J.E., A. Malkassian, R. Kudela, E. Novick, M. Peacock, T. Schraga, and D. Senn. 2014. The Suisun Bay problem: Food quality or food quantity? Interagency Ecological Program Newsletter 27 (1): 15–23.
Dege, M., and L.R. Brown. 2004. Effect of outflow on spring and summertime distribution and abundance of larval and juvenile fishes in the upper San Francisco Estuary. American Fisheries Society Symposium 39: 49–65.
Dill, L.M., and A.H.G. Fraser. 1984. Risk of predation and the feeding behavior of juvenile Coho salmon (Oncorhynchus kisutch). Behavioral Ecology and Sociobiology 16 (1): 65–71.
Durant, J.M., D. Hjermann, G. Ottersen, and N.C. Stenseth. 2007. Climate and the match or mismatch between predator requirements and resource availability. Climate Research 33: 271–283.
Feyrer, F., B. Herbold, S.A. Matern, and P.B. Moyle. 2003. Dietary shifts in a stressed fish assemblage: Consequences of a bivalve invasion in the San Francisco Estuary. Environmental Biology of Fishes. 67: 277–288.
Garwood, R.S. 2017. Historic and contemporary distribution of Longfn Smelt (Spirinchus thaleichthys) along the California coast. California Fish and Game 103 (3): 96–117.
Grimaldo, L., J. Burns, R. E. Miller, A. Kalmbach, A. Smith, J. Hassrick, and C. Brennan. 2020. Forage fish larvae distribution and habitat use during contrasting years of low and high freshwater flow in the San Francisco estuary. San Francisco Estuary and Watershed Science 18(3). https://doi.org/10.15447/sfews.2020v18iss3art5
Grimaldo, L., F. Feyrer, J. Burns, and D. Maniscalco. 2017. Sampling uncharted waters: Examining rearing habitat of larval Longfin Smelt (Spirinchus thaleichthys) in the Upper San Francisco Estuary. Estuaries and Coasts 40: 1771–1784.
Hammock, B.G., S.P. Moose, S.S. Solis, E. Goharian, and S.J. Teh. 2019. Hydrodynamic modeling coupled with long-term field data provide evidence for suppression of phytoplankton by invasive clams and freshwater exports in the San Francisco Estuary. Environmental Management 63: 703–717.
Hennessy, A. 2018. Zooplankton monitoring 2017. Interagency Ecological Program Newsl. 32 (1): 21–32.
Herbold, B., S. M. Carlson, R. Henery, R. C. Johnson, N. Mantua, M. McClure, P. Moyle, and T. Sommer. 2018. Managing for salmon resilience in California’s variable and changing climate. San Francisco Estuary and Watershed Science 16.
Hobbs, J.A., W.A. Bennett, and J.E. Burton. 2006. Assessing nursery habitat quality for native smelts (Osmeridae) in the low-salinity zone of the San Francisco estuary. Journal of Fish Biology 69: 907–922.
Holling, C.S. 1959. The components of predation as revealed by a study of small-mammal predation of the European Pine Sawfly. The Canadian Entomologist, 91(5), 293–320.Houde, E. D. 1975. Effects of stocking density and food density on survival, growth and yield of laboratory-reared larvae of sea bream Archosargus rhomboidalis (L.) (Sparidae). Journal of Fish Biology 7: 115–127.
Houde, E.D. 1978. Critical food concentrations for larvae of three species of subtropical marine fishes. Bulletin of Marine Science 28: 395–411.
Houde, E.D. 1987. Fish early life dynamics and recruitment variability. American Fisheries Society Symposium 2: 17–29.
Hutton, P.H., J.S. Rath, and S.B. Roy. 2017a. Freshwater flow to the San Francisco Bay-Delta estuary over nine decades (Part 1): Trend evaluation. Hydrological Processes 31: 2500–2515.
Hutton, P.H., J.S. Rath, and S.B. Roy. 2017b. Freshwater flow to the San Francisco Bay-Delta estuary over nine decades (Part 2): Change attribution. Hydrological Processes 31: 2516–2529.
Jungbluth, M.J., J. Burns, L. Grimaldo, A. Slaughter, A. Katla, and W. Kimmerer. 2021. Feeding habits and novel prey of larval fishes in the northern San Francisco Estuary. Environmental DNA 00: 1–22.
Kimmerer, W.J. 2002. Effects of freshwater flow on abundance of estuarine organisms: Physical effects or trophic linkages. Marine Ecology Progress Series 243: 39–55.
Kimmerer, W.J. 2006. Response of anchovies dampens effects of the invasive bivalve Corbula amurensis on the San Francisco Estuary foodweb. Marine Ecology Progress Series 324: 207–218.
Kimmerer, W.J., E.S. Gross, and M.L. MacWilliams. 2009. Is the response of estuarine nekton to freshwater flow in the San Francisco Estuary explained by variation in habitat volume? Estuaries and Coasts 32: 375–389.
Kimmerer, W. J., Gross, E. S., Slaughter, A. M., and Durand, J. R. 2018. Spatial subsidies and mortality of an estuarine copepod revealed using a box model. Estuaries and Coasts.
Kristiansen, T., K. F. Drinkwater, R. G. Lough, and S. Sundby. 2011. Recruitment variability in North Atlantic cod and match-mismatch dynamics. PLoS One 6.
Lefcheck, J.S., B.B. Hughes, A.J. Johnson, B.W. Pfirrmann, D.B. Rasher, A.R. Smyth, B.L. Williams, M.W. Beck, and R.J. Orth. 2019. Are coastal habitats important nurseries? A meta-analysis. Conservation Letters 12: 1–12.
Levy, L., A. Liberzon, T. Elmaliach, and R. Holzman. 2017. Hydrodynamic regime determines the feeding success of larval fish through the modulation of strike kinematics. Proceedings of the Royal Society 284: 20170235.
Lewis, Levi, A. Barros, M. Willmes, C. Denney, C. Parker, M.Bisson, J. Hobbs, A. Finger, G. Benjamin, A. Benjamin. 2019. Interdisciplinary studies on Longfin Smelt in the San Francisco Estuary. https://doi.org/10.13140/RG.2.2.12944.33280.
Lewis, L. S., M. Willmes, A. Barros, P. K. Crain, and J. A. Hobbs. 2020. Newly discovered spawning and recruitment of threatened Longfin Smelt in restored and under-explored tidal wetlands. Ecology 0:4–7.
Liu, Q., F. Chai, R. Dugdale, Y. Chao, H. Xue, S. Rao, F. Wilkerson, J. Farrara, H. Zhang, Z. Wang, and Y. Zhang. 2018. San Francisco Bay nutrients and plankton dynamics as simulated by a coupled hydrodynamic-ecosystem model. Continental Shelf Research 161: 29–48.
Lotze, H.K., H.S. Lenihan, B.J. Bourque, R.H. Bradbury, R.G. Cooke, M.C. Kay, S.M. Kidwell, M.X. Kirby, C.H. Peterson, and J.B.C. Jackson. 2006. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312: 1806–1809.
Lusardi, R.A., B.G. Hammock, C.A. Jeffres, R.A. Dahlgren, and J.D. Kiernan. 2019. Oversummer growth and survival of juvenile coho salmon (Oncorhynchus kisutch) across a natural gradient of stream water temperature and prey availability: An in situ enclosure experiment. Canadian Journal of Fisheries and Aquatic Sciences 12: 1–12.
Macvean, L.J., and M.T. Stacey. 2011. Estuarine dispersion from tidal trapping: A new analytical framework. Estuaries and Coasts 34: 45–59.
Malone, T.C., L.H. Crocker, S.E. Pike, and B.W. Wendler. 1988. Influences of river flow on the dynamics of phytoplankton production in a partially stratified estuary. Marine Ecology 48: 235–249.
Mann, M.E., and P.H. Gleick. 2015. Climate change and California drought in the 21st century. Proceedings of the National Academy of Sciences of the United States of America 112 (13): 3858–3859.
Meekan, M.G., L. Vigliola, A. Hansen, P.J. Doherty, A. Halford, and J.H. Carleton. 2006. Bigger is better: Size-selective mortality throughout the life history of a fast-growing clupeid, Spratelloides gracilis. Marine Ecology Progress Series 317: 237–244.
Minchin, P.R. 1987. An Evaluation of the Relative Robustness of Techniques for Ecological Ordination. Plant Ecology 69: 89–107.
Moore, J.W., and I.A. Moore. 1976. The basis of food selection in flounders, Platichthys flesus (L.), in the Severn Estuary. Journal of Fish Biology 9: 139–156.
Moyle, P. 2002. Inland fishes of California. Revised and Expanded Edition: Page University of California Press.
Nichols, F.H., J.E. Cloern, S.N. Luoma, and D.H. Peterson. 1986. The modification of an estuary. Science 231: 567–573.
Nobriga, M.L., and J.A. Rosenfield. 2016. Population dynamics of an estuarine forage fish: Disaggregating forces driving long-term decline of Longfin smelt in California’s San Francisco estuary. Transactions of the American Fisheries Society 145 (1): 44–58.
Odum, E.P. 1961. The role of tidal marshes in estuarine production. New York State Conservation 15: 12–15.
Oksanen, J., F.G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P.R. Minchin, R. O’Hara, G.L. Simpson, P. Solymos, M.H.H. Stevens, E. Szoecs, and H. Wagner. 2019. vegan, R.
Prentice, I.C. 1977. Non-metric ordination methods in ecology. Journal of Ecology 65: 85–94. https://www.jstor.org/stable/2259064
Rose, K.A., W.J. Kimmerer, K.P. Edwards, and W.A. Bennett. 2013. Individual-based modeling of delta smelt population dynamics in the upper san francisco estuary: II. alternative baselines and good versus bad years. Transactions of the American Fisheries Society 142: 1260–1272.
Rosenfield, J.A., and R.D. Baxter. 2007. Population dynamics and distribution patterns of Longfin Smelt in the San Francisco Estuary. Transactions of the American Fisheries Society 136: 1577–1592.
Rypel, A.L., C.A. Layman, and D.A. Arrington. 2007. Water depth modifies relative predation risk for a motile fish taxon in Bahamian Tidal Creeks. Estuaries and Coasts 30: 518–525.
Sağlam, İK., J. Hobbs, R. Baxter, L.S. Lewis, A. Benjamin, and A.J. Finger. 2021. Genome-wide analysis reveals regional patterns of drift, structure, and gene flow in longfin smelt ( Spirinchus thaleichthys ) in the northeastern Pacific. Canadian Journal of Fisheries and Aquatic Sciences 12 (May): 1–12.
Scavia, D., J.C. Field, D.F. Boesch, R.W. Buddemeier, V. Burkett, D.R. Cayan, M. Fogarty, M.A. Harwell, R.W. Howarth, C. Mason, D.J. Reed, T.C. Royer, A.H. Sallenger, and J.G. Titus. 2002. Climate change impacts on U.S. coastal and marine ecosystems. Estuaries 25: 149–164.
Schelske, C., and E. Odum. 1962. Mechanisms maintaining high productivity in Georgia estuaries. Page Estuarine Session. Washington D.C.
Senn, D., and M. Sutula. 2015. Scientific basis to assess the effects of nutrients on San Francisco Bay beneficial uses.
Sheaves, M., R. Baker, I. Nagelkerken, and R.M. Connolly. 2014. True value of estuarine and coastal nurseries for fish: Incorporating complexity and dynamics. Estuaries and Coasts 38: 401–414.
SJSCRWF. 2015. Annual Self Monitoring Report. San Jose-Santa Clara Regional Wastewater Facility. San Jose, CA.
Slater, S.B., and R. Baxter. 2014. Diet, prey selection, and body condition of Age-0 Delta Smelt, Hypomesus transpacificus, in the Upper San Francisco Estuary. San Francisco Estuary & Watershed Science 12: 1–24.
Sommer, T., C. Armor, R. Baxter, R. Breuer, L. Brown, M. Chotkowski, S. Culberson, F. Feyrer, M. Gingras, B. Herbold, W. Kimmerer, A. Mueller-Solger, M. Nobriga, and K. Souza. 2007. The collapse of pelagic fishes in the Upper San Francisco Estuary. Fisheries 32: 270–277.
Team, R. C. 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Thebault, J., T. Schraga, J. Cloern, and E. Dunlavey. 2008. Primary production and carrying capacity of former salt ponds after reconnection to San Francisco Bay. Wetlands 28: 841–851.
Valoppi, L. 2018. Phase 1 studies summary of major findings of the South Bay Salt Pond Restoration Project, South San Francisco Bay, California: U.S. Geological Survey Open-File Report 2018–1039, 58 p.
Vasconcelos, R.P., P. Reis-Santos, M.J. Costa, and H.N. Cabral. 2011. Connectivity between estuaries and marine environment: Integrating metrics to assess estuarine nursery function. Ecological Indicators 11: 1123–1133.
Walters, R.A., R.T. Cheng, and T.J. Conomos. 1985. Time scales of circulation and mixing processes of San Francisco Bay waters. Hydrobiologia 129: 13–36.
Wang, J. C. S. 1986. Fishes of the Sacramento-San Joaquin estuary and adjacent waters, California : A guide to the early life histories. Interagency Ecological Program Technical Report.
Whipple, A. A., Grossinger, R. M., Rankin, D., Stanford, B., and Askevold, R. A. 2012. Sacramento-San Joaquin Delta historical ecology investigation: Exploring pattern and process. Prepared for the California Department of Fish and Game and Ecosystem Restoration Program A Report of SFEI-ASC’s Historical Ecology Program, Publication #672, August, 408. San Francisco Estuary Institute-Aquatic Science Center, Richmond, CA.
Williams, P.B., and M.K. Orr. 2002. Physical evolution of restored breached levee salt marshes in the San Francisco Bay estuary. Restoration Ecology 10: 527–542.
Winder, M., and A.D. Jassby. 2011. Shifts in zooplankton community structure: Implications for food web processes in the Upper San Francisco Estuary. Estuaries and Coasts 34: 675–690.
Acknowledgements
We would like to thank the many students and staff in the Otolith Geochemistry and Fish Ecology Laboratory, especially A. Alfonso, A. Zaydahr-Kulka, A. Huynh, and D. Conway, for assistance with field collections and laboratory processing of fish and zooplankton samples. We also thank the California Department of Fish and Wildlife Zooplankton Study for providing data on the upper estuary, including Tricia Bippus for providing training on larval fish dissections and zooplankton identification and Randy Baxter for providing valuable feedback. We also thank several anonymous reviewers for providing feedback that greatly improved the manuscript.
Funding
Funding for this study was provided by grants to J. Hobbs and L. Lewis from the California Department of Water Resources (Grant No. 4600011196) and the US Fish and Wildlife Service (Grant No. F18AC00058). Andrew L. Rypel was supported by the Peter B. Moyle & California Trout Endowment for Coldwater Fish Conservation. Funding was also provided by the California Agricultural Experimental Station of the University of California Davis, grant number CA-D-WFB-2467-H to ALR and CA-D-WFB-2098-H to Nann A. Fangue.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David G. Kimmel
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barros, A., Hobbs, J.A., Willmes, M. et al. Spatial Heterogeneity in Prey Availability, Feeding Success, and Dietary Selectivity for the Threatened Longfin Smelt. Estuaries and Coasts 45, 1766–1779 (2022). https://doi.org/10.1007/s12237-021-01024-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-021-01024-y