Abstract
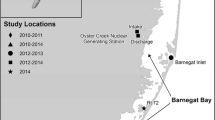
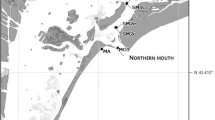
Throughout the Gulf of Mexico (GoM), coastal inlets provide vital pathways for marine fish larvae spawned offshore to reach estuarine nursery habitat. While survivorship of fish larvae is dependent on many factors, feeding success has been highlighted as the most important. Currently, little is known about the feeding ecology of larval fish during their passage through coastal inlets. The purpose of this study was to investigate the trophodynamics of fish larvae passing through a GoM estuarine tidal inlet during a fall spawning season. Several trophic niches existed within the δ13C’ and δ15N’ (lipid and baseline corrected values) stable isotope-derived food web of larval fish, with multiple taxa present within most trophic niches. These trophic niches changed throughout the fall season, however. Micropogonias undulatus, an estuarine-dependent species common to the region, was selected for a closer look at dietary shifts through ontogeny and showed an overall shift to higher trophic level prey with size, although there was not yet evidence of a shift towards estuarine prey indicative of settlement. Their diet mainly consisted of Calanoida copepods across all sizes of larvae with significant electivity for only Acartia (Calanoida), and this selection was likely due to a difference in behavior rather than taxonomy or size. This study provides information about larval fish trophodynamics at coastal inlets, advancing our understanding of larval fish survivorship and recruitment to adult fish stocks.





Similar content being viewed by others
References
Boltovskoy, D. 1999. South Atlantic zooplankton. Netherlands: Backhuys Publishers.
Bradley, C.J., J.R. Strickler, E.J. Buskey, and P.H. Lenz. 2013. Swimming and escape behavior in two species of calanoid copepods from nauplius to adult. Journal of Plankton Research 35 (1): 49–65.
Brown, C.A., S.A. Holt, G.A. Jackson, D.A. Brooks, and G.J. Holt. 2004. Simulating larval supply to estuarine nursery areas: How important are physical processes to the supply of larvae to the Aransas Pass Inlet? Fisheries Oceanography 13 (3): 181–196.
Brown, S.C., J.J. Bizzarro, G.M. Cailliet, and D.A. Ebert. 2012. Breaking with tradition: Redefining measures for diet description with a case study of the Aleutian skate Bathyraja aleutica (Gilbert 1896). Environmental Biology of Fishes 95 (1): 3–20.
Buskey, E.J., K.H. Dunton, and P.L. Parker. 1999. Variations in stable carbon isotope ratio of the copepod Acartia tonsa during the onset of the Texas brown tide. Estuaries 22 (4): 995–1003.
Cervetto, G., R. Gaudy, and M. Pagano. 1999. Influence of salinity on the distribution of Acartia tonsa (Copepoda, Calanoida). Journal of Experimental Marine Biology and Ecology 239: 33–45.
Chesson, J. 1978. Measuring preference in selective predation. Ecology 59 (2): 211–215.
Costello, J.H., R. Loftus, and R. Waggett. 1999. Influence of prey detection on capture success for the ctenophore Mnemiopsis leidyi feeding upon adult Acartia tonsa and Oithona colcarva copepods. Marine Ecology Progress Series 191: 207–216.
Fahay, M.P. 2007. Early stages of fishes in the western North Atlantic Ocean. Canada: Dartmouth.
Fortier, L., and R. Harris. 1989. Optimal foraging and density-dependent competition in marine fish larvae. Marine Ecology Progress Series 51: 19–33.
Forward, R., Jr., K. Reinsel, D. Peters, R. Tankersley, J. Churchill, L. Crowder, W. Hettler, S. Warlen, and M. Green. 1999. Transport of fish larvae through a tidal inlet. Fisheries Oceanography 8: 153–172.
Froeschke, J.T., and B.F. Froeschke. 2016. Two-stage boosted regression tree model to characterize southern flounder distribution in Texas estuaries at varying population sizes. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science 8: 222–231.
Fry, B. 2006. Stable isotope ecology. New York: Springer.
Fry, B., and E.B. Sherr. 1984. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contributions in Marine Science 27: 13–47.
Geist, S.J., A. Kunzmann, H.M. Verheye, A. Eggert, A. Schukat, and W. Ekau. 2015. Distribution, feeding behaviour, and condition of Cape horse mackerel early life stages, Trachurus capensis, under different environmental conditions in the northern Benguela upwelling ecosystem. ICES Journal of Marine Science 72 (2): 543–557.
Gemmel, B.J., and E.J. Buskey. 2011. The transition from nauplii to copepodites: Susceptibility of developing copepods to fish predators. Journal of Plankton Research 33 (11): 1773–1777.
Govoni, J.J. 1997. The association of the population recruitment of gulf menhaden, Brevoortia patronus, with Mississippi River discharge. Journal of Marine Systems 12: 101–108.
Govoni, J.J., D.E. Hoss, and A.J. Chester. 1983. Comparative feeding of three species of larval fishes in the Northern Gulf of Mexico: Brevoortia patronus, Leiostomus xanthurus, and Micropogonias undulatus. Marine Ecology Progress Series 13: 189–199.
Govoni, J.J., P.B. Ortner, F. Al-Yamani, and L.C. Hill. 1986. Selective feeding of spot, Leiostomus xanthurus, and Atlantic croaker, Micropogonias undulatus, larvae in the northern Gulf of Mexico. Marine Ecology Progress Series 28: 175–183.
Govoni, J.J., and A.J. Chester. 1990. Diet composition of larval Leiostomus xanthurus in and about the Mississippi River plume. Journal of Plankton Research 12 (4): 819–830.
Greening, H., P. Doering, and C. Corbett. 2006. Hurricane impacts on coastal ecosystems. Estuaries and Coasts 29 (6): 877–879.
Gunter, G. 1945. Studies on marine fishes of Texas. Publications of the Institute of Marine Science Texas 1: 45–46.
Hall, Q.A., M.M. Reese Robillard, J.A. Williams, M.J. Ajemian, and G.W. Stunz. 2016. Reopening of a remote tidal inlet increases recruitment of estuarine-dependent nekton. Estuaries and Coasts 39 (6): 1769–1784.
Herzka, S.Z., S.A. Holt, and G.J. Holt. 2001. Documenting the settlement history of individual fish larvae using stable isotope ratios: Model development and validation. Journal of Experimental Marine Biology and Ecology 265: 49–74.
Holt, S.A., G.J. Holt, and C.R. Arnold. 1990. Abundance and distribution of larval fishes and shrimps in the Laguna Madre, Texas: a hypersaline lagoon. Texas Water Development Board Technical Report No. TR/90–007.
Holt, G.J., and S.A. Holt. 2000. Vertical distribution and the role of physical processes in the feeding dynamics of two larval sciaenids Sciaenops ocellatus and Cynoscion nebulosus. Marine Ecology Progress Series 193: 181–190.
Houde, E.D. 1987. Fish early life dynamics and recruitment variability. American Fisheries Society Symposium 2: 17–29.
Houde, E.D. 2008. Emerging from Hjort’s shadow. Journal of Northwest Fisheries Science 41: 53–70.
Hunter, J.R. 1984. Feeding ecology and predation of marine fish larvae. In Marine fish larvae, ed. R. Lasker, 33–77. Seattle: University of Washington Press.
Jackson, J.M., and P.H. Lenz. 2016. Predator-prey interactions in the plankton: Larval fish feeding on evasive copepods. Scientific Reports 6: 33585.
Johnson, W.S., and D.M. Allen. 2012. Zooplankton of the Atlantic and Gulf coasts: A guide to their identification and ecology. Maryland: Johns Hopkins University Press.
Kupchik, M.J., and R.F. Shaw. 2016. Age, growth, and recruitment of larval and early juvenile Atlantic croaker (Micropogonias undulatus), determined from analysis of otolith microstructure. Fishery Bulletin 114 (1): 18–33.
Lemley, D.A., J.B. Adams, T.G. Bornman, E.E. Campbell, and S.H.P. Deyzel. 2019. Land-derived inorganic nutrient loading to coastal waters and potential implications for nearshore plankton dynamics. Continental Shelf Research 174: 1–11.
Levinton, J., M. Doall, D. Ralston, A. Starke, and B. Allam. 2011. Climate change, precipitation and impacts on an estuarine refuge from disease. Plos One 6(4): e18849.
Liu, H., X. Zhang, Q. Yang, T. Zuo, and A. Quigg. 2017. Mesozooplankton dynamics in relation to environmental factors and juvenile fish in a subtropical estuary of the Gulf of Mexico. Journal of Coastal Research 33 (5): 1038–1050.
Madeira, D., L. Narciso, H.N. Cabral, and C. Vinagre. 2012. Thermal tolerance and potential impacts of climate change on coastal and estuarine organisms. Journal of Sea Research 70: 32–41.
McConnaughey, T., and C.P. McRoy. 1979. Food-web structure and the fractionation of carbon isotopes in the Bering Sea. Marine Biology 53: 257–262.
Mitra, A. and S. Zaman. 2016. Threats to marine and estuarine ecosystems. In Basics of Marine and Estuarine Ecology, 365–417. New Delhi: Springer.
Nakayama, S., and L.A. Fuiman. 2010. Body size and vigilance mediate asymmetric interference competition for food in fish larvae. Behavioral Ecology 21 (4): 708–713.
Paris, C.B., and R.K. Cowen. 2004. Direct evidence of a biophysical retention mechanism for coral reef fish larvae. Limnology and Oceanography 49 (6): 1964–1979.
Pepin, P., and J.F. Dower. 2007. Variability in the trophic position of larval fish in a coastal pelagic ecosystem based on stable isotope analysis. Journal of Plankton Research 29 (8): 727–737.
Pepin, P., and R. Penney. 2000. Feeding by a larval fish community: Impact on zooplankton. Marine Ecology Progress Series 204: 199–212.
Peterson, B.J., and B. Fry. 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18 (1): 293–320.
Petrik, C.M., T. Kristiansen, G.R. Lough, and C.S. Davis. 2009. Prey selection by larval haddock and cod on copepods with species-specific behavior: An individual-based model analysis. Marine Ecology Progress Series 396: 123–143.
Reese, M.M., G.W. Stunz, and A.M. Bushon. 2008. Recruitment of estuarine-dependent nekton through a new tidal inlet: The opening of Packery Channel in Corpus Christi, TX, USA. Estuaries and Coasts 31: 1143–1157.
Richards, W.J. 2005. Early stages of Atlantic fishes: An identification guide for the Western Central North Atlantic. Florida: CRC Press.
Rijnsdorp, A.D., M.V. Stralen, and H.W. Van Der Veer. 1985. Selective tidal transport of North Sea plaice larvae Pleuronectes platessa in coastal nursery areas. Transactions of the American Fisheries Society 114 (4): 461–470.
Rooker, J.R., and S.A. Holt. 1997. Utilization of subtropical seagrass meadows by newly settled red drum Sciaenops ocellatus: Patterns of distribution and growth. Marine Ecology Progress Series 158: 139–149.
Schaffler, J.J., C.S. Reiss, and C.M. Jones. 2009. Spatial variation in otolith microchemistry of Atlantic croaker larvae in the Mid-Atlantic Bight. Marine Ecology Progress Series 382: 185–195.
Schukat, A., H. Auel, N. Lahajnar, and W. Hagen. 2014. Complex trophic interactions of calanoid copepods in the Benguela upwelling system. Journal of Sea Research 85: 186–196.
Soto, M.A., G.J. Holt, S.A. Holt, and J.R. Rooker. 1998. Food habits and dietary overlap of newly settled red drum (Sciaenops ocellatus) and Atlantic croaker (Micropogonias undulatus) from Texas seagrass meadows. Gulf Research Reports 10: 41–55.
Thayer, G.W., J.J. Govoni, and D.W. Connally. 1983. Stable carbon isotope ratios of the planktonic food web in the northern Gulf of Mexico. Bulletin of Marine Science 33 (2): 247–256.
Tunnell, J.W., K. Withers, E.H. Smith, F.W. Judd, J.F. Bergan, N.L. Hilbun, A.E. Koltermann, S.J. Dilworth, and S.A. Childs. 2002. The Laguna Madre of Texas and Tamaulipas. College Station: Texas A&M University Press.
Vaslet, A., C. France, D.L. Phillips, I.C. Feller, and C.C. Baldwin. 2011. Stable-isotope analyses reveal the importance of seagrass beds as feeding areas for juveniles of the speckled worm eel Myrophis punctatus (Teleostei: Ophichthidae) in Florida. Journal of Fish Biology 79: 692–706.
Waggett, R.J., and E.J. Buskey. 2007. Copepod escape behavior in non-turbulent and turbulent hydrodynamic regimes. Marine Ecology Progress Series 334: 193–198.
Welker, M.T., C.L. Pierce, and D.H. Wahl. 1994. Growth and survival of larval fishes: Roles of competition and zooplankton abundance. Transactions of the American Fisheries Society 123: 703–717.
Acknowledgements
Fish collection was conducted under TPWD Scientific Permit SPR-0316-065. We would like to thank the Texas A&M University-Corpus Christi Fisheries and Mariculture program for providing additional research funds. Thank you to Dr. Jim Tolan for advising and providing feedback on this text and throughout the project. Thank you to Dr. Benoit Lebreton and Dr. Paula Rose for their aid in stable isotope sample processing. Special thanks to Stormy Paxton, Jason Selwyn, Dr. Sterba-Boatwright, Chriss Shope, Polly Hajovsky, Shannan McAskill, Corbyn Porter, Daniel Hardin, Valeria Nunez, Lisa Gignac, Natasha Breaux, Melissa Rohal, Ashleigh Campbell, Cody Myers, Cristian Camacho, and Elizabeth Hunt for their help in field collections and sample processing.
Funding
This research was funded by the Texas A&M University-Corpus Christi startup funds of S. Geist and followed ethical compliance standards (TAMUCC-AUP 03–16).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David G. Kimmel
Rights and permissions
About this article
Cite this article
Bromschwig, M.J., Geist, S.J. Feeding Ecology of a Larval Fish Assemblage During its Passage Through a Coastal Inlet in the Northwestern Gulf of Mexico. Estuaries and Coasts 45, 866–881 (2022). https://doi.org/10.1007/s12237-021-01002-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-021-01002-4