Abstract
Intertidal crabs display distinct morphological traits that allow differential interactions with biotic and abiotic features of the intertidal landscape, but are also influenced by allometry and sexual selection. This study aimed to explore the influence of sexual, allometric and habitat factors on morphological variation in the intertidal mangrove crab assemblage. A standardized photographic protocol was developed using readily available, low-cost technology to capture the morphology of carapaces and claws as sets of Cartesian landmarks. Digitization errors were 1 to 2 orders of magnitude smaller than the variation among individuals. In Tubuca seismella and Tubuca signata (superfamily Ocypodoidea), species that have traditionally been studied for sexual dimorphism, standardized major claw sizes were 2.8 and 3.7, respectively, times larger for males than females. Sexual dimorphism in claw size was also observed for Metopograpsus frontalis and Parasesarma longicristatum (superfamily Grapsoidea), with the largest claw in males being 15% and 33%, respectively, larger than in females. In contrast to size, claw shape did not relate to sex, except for T. seismella. Carapace shape, although variable among individuals and displaying asymmetry, was unrelated to sex. Claw and carapace shapes displayed high correlations, with values around 0.78. Carapace shapes grouped into taxonomic families and linked to habitat preferences, while claw shapes varied along a taxonomic gradient. These results complement studies on crab morphology that focus on specific factors or species, and stress the importance of multiple, interacting factors including sexual, allometric and habitat influences as drivers of morphological trait variability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intertidal crabs form a fundamental component of macro-benthic assemblages in (sub)tropical estuaries that is closely linked to the wider ecosystem via trophic interactions and ecosystem engineering. Intertidal crabs influence, for instance, sediment composition (Botto and Iribarne 1999; Escarpa et al. 2004), productivity (Koch and Wolff 2002; Werry and Lee 2005), vegetation structure (Bosire et al. 2005), faunal composition (Dye and Lasiak 1986; Botto et al. 2000) and energy fluxes (Wolff et al. 2000). Grapsoidea and Ocypodoidea are dominant superfamilies in the intertidal zone of (sub)tropical estuaries (Lee 2008; Nagelkerken et al. 2008). Species in each of these superfamilies have distinct morphological traits allowing differential interactions with biotic and abiotic features of the intertidal landscape. For instance, the long-stalked eyes of many species in the superfamily Ocypodoidea allow them to maintain a low posture while still being able to scope out their surroundings (Zeil and Hemmi 2006). The morphology of their eyes is specifically adapted to discern between predators in the upper part of their field of view and social signals of other crabs in the lower part. This adaptation is effective in flat, low-structure habitats where the distribution of predators and co-specifics is organized along a vertical axis (Zeil and Hemmi 2006). Meanwhile, the position of the eyes in the families Grapsidae and Sesarmidae (superfamily Grapsoidea) at each lateral boundary of their carapace (termed ‘broad-fronted’ species) allows for well-developed stereoscopic vision (Zeil and Hemmi 2006). This allows them to gauge distances and to move efficiently through habitats with high structural complexity, such as dense mangrove roots (Zeil et al. 1986).
The morphology of organisms, however, is also influenced by genetic factors, allometry, phylogenetic constraints and sexual selection (Parsons 1992; Voje 2016). For example, the fiddler crabs (Subfamily: Gelasiminae) that are prevalent in many (sub)tropical estuaries (Nagelkerken et al. 2008) depend strongly on visual communication strategies such as claw waving and changes in body posture (Zeil and Hemmi 2006; How et al. 2008). The attraction of mates using visual signals has resulted in strong sexual dimorphism, with males having one enlarged claw while their female counterparts have two smaller claws similar in size. This sexual dimorphism has made fiddler crabs the topic of several behavioural studies (e.g. Valiela et al. 1974; Oliveira and Custodio 1998; Allen and Levinton 2007) and is a factor when linking morphological traits to habitat preference patterns. In fact, the essential use of visual signals in sexual communication within this genus could restrict their spatial distribution to habitats with little structural complexity, particularly for small fiddler crabs with highly developed waving displays such as Tubuca seismella (von Hagen 1993).
Characterization of organisms’ shapes has often been undertaken using measurements such as length and height of morphological features. Such measurements are inherently linked to the size of the specimens being investigated (Zelditch et al. 2012). This limitation can be overcome by calculating ratios of different measurements, which provide more comparable metrics and allow intra- and interspecific comparisons. Nonetheless, ratios inflate small measurement errors and provide little information on the overall geometric structure of morphological features (Zelditch et al. 2012). An alternative method involves combining individual measurements into a network of angles and measurements, generating a detailed morphometric profile (Bookstein et al. 1985). This method, however, does not lend itself well to statistical analysis, as networks are difficult to capture within a single, parsimonious set of measurements. Geometric morphometrics offers a solution by characterising shapes as a single set of Cartesian landmarks (also called coordinates), preserving the shape of an individual organism independent of its size and thus allowing for transformation, rotation, resizing and subsequent inclusion in statistical analysis (Zelditch et al. 2012).
Digital imaging technologies enable rapid and cost-effective collection of geometric landmarks and have previously been used to compare shape variations of crabs (Rosenberg 2002; Hopkins and Thurman 2010; Hampton et al. 2014). Nonetheless, accurate collection of geometric landmarks requires careful assessment of measurement errors induced by imaging and digitization, especially when obtaining two-dimensional data from three-dimensional objects (Cardini 2014). Random measurement errors inflate the variance in a dataset which reduces statistical power in analyses that are based on assessments of explained versus residual variance (Fruciano 2016). Meanwhile, systematic measurement errors can bias results and lead to misinterpretation of this bias as a biologically meaningful pattern (Fruciano 2016). Measurement errors are particularly relevant when assessing subtle shape changes such as variations in the level of asymmetry which are relatively small compared to variation among individuals, sexes or species, making it easier for variations in asymmetry to be masked by measurement error (Klingenberg 2009). Nonetheless, geometric morphometrics can be successfully applied in many cases. For instance, the error due to image collection and digitization was more than 41 times smaller than variation in fluctuating asymmetry and among individuals in the wing shape of the fly Drosophila melanogaster, and had a negligible effect on the analyses performed (Breuker et al. 2006). The relative influence of measurement error, however, depends on the organism and body part under investigation (Fruciano 2016). Consequently, validated, standardized protocols for the collection of landmark sets for geometric morphometrics are needed before this approach can be used for accurate ecological studies.
The present study aimed to explore the relative influence of sexual, allometric and habitat-related influences on morphological trait variation in the intertidal mangrove crab assemblage characterized by species with strong morphological sexual dimorphism. The specific objectives were to (1) quantify the strength of allometric and sexual influences on intraspecific shape and size variation, and (2) relate morphological variation within the intertidal crab assemblage to preferences of species for habitats with different characteristics (including tidal height, bank angle, vegetation and canopy cover). To support these morphometric analyses, the study developed and validated a standardised protocol for the collection of shape information regarding crab carapaces and claws suitable for geometric morphometric analyses.
Materials and Methods
Study Sites and Field Collection
Ross River Estuary and Pallarenda Creek located near Townsville, Queensland, Australia (19°16′ 00′′ S, 146° 49′ 20″ E, Fig. 1) were chosen as study sites since these estuaries have different habitats within their intertidal landscapes and various crab species inhabiting them.
A biodiversity survey was carried out by Vermeiren (2013) in 2011 at four locations across the Ross River Estuary and Pallarenda Creek (Fig. 1) to assess the preferences of different crab species for distinct habitats within the intertidal landscape. The data collected during the survey were used to explore links between morphological traits of crabs and their habitat preferences. An in-depth evaluation of species distributions in relation to habitat preferences was beyond the scope of the current study but can be found in Vermeiren (2013) and Vermeiren and Sheaves (2015a, b). Briefly, habitats at each of the four locations were identified based on a classification into three distinct zones with characteristic tidal elevation-vegetation pattern combinations. These zones were further divided based on dominant vegetation types leading to a total of nine distinct habitats. The three zones included low intertidal banks, devoid of any vegetation, extending from mean low water during spring tidal cycles to the edge of the mangrove forest; mid-intertidal mangrove forests extending from the water-ward edge of the forest towards mean high water at neap tidal cycles; and high intertidal flats extending above mean high water at neap tidal cycles and devoid of mangrove forests. The nine habitats identified across the three zones were low intertidal banks bordered by Ceriops spp.; low intertidal banks bordered by Rhizophora stylosa; low intertidal banks bordered by Avicennia marina; low intertidal banks without adjacent vegetation; mid-intertidal Ceriops spp. forest; mid-intertidal R. stylosa forest; mid-intertidal A. marina forest; high intertidal flats with saltmarsh Sporobolus spp.; and bare saltpans in the high intertidal. Habitats at each site within each location were characterized by the following environmental factors to allow a first exploration of morphology-environment linkages within the scope of the current study: vegetation (dominant species of vegetation or ‘bare’), tidal height (‘low’, ‘mid’ and ‘high’ following the definition of the zones), and continuous variables for bank angle (in degrees) and canopy cover (as percentage).
The relative abundance of intertidal crab species was assessed using a stratified sampling design with three random replicate sites for each habitat in location 1, and two random replicate sites for each habitat in locations 2, 3 and 4. Hand catches of crabs active at the surface or hiding at their burrow entrances were conducted for 5 min per site with the aid of a shovel to block burrow entrances (Macintosh et al. 2002; Castiglioni and Negreiros-Fransozo 2006; Vermeiren and Sheaves 2014). Sampling took place during ebb spring tide on 17 and 21 May 2011 at location 1 and on 3, 5 and 16 April 2011 at locations 2, 3 and 4, respectively. Ross River Estuary and Pallarenda Creek are subject to semidiurnal tides, with tidal ranges near the estuary mouth around 3 m on spring tides. Salinities within the estuary channel at the specific sampling times and locations were near local sea water salinity of 35 ppt, which is consistent with the salinity profile of these small, tide-dominated dry tropical estuaries after the wet season (Sheaves et al. 2010). All crabs caught were identified in the field to species level (following Campbell 1977; Frusher 1988 and Poore 2004; with their taxonomy crosschecked against the WoRMS database; Horton et al. 2020) and released after identification (Table 1). Seven individuals were identified as either Metopograpsus thukuhar or Austruca perplexa, yet their identification was considered unreliable as they were small juveniles. Moreover, individuals of these two species contributed < 1% to the total number of crabs caught. These two species were therefore removed from subsequent analyses.
In addition to the biodiversity survey, adults (male and female) from different species were also collected to establish morphological variation in carapace and claw shapes within and among intertidal mangrove crabs, and to assist in developing a standardized protocol to collect landmark data for geometric morphometric analyses. Specifically, crab species were hand-caught across the intertidal zone throughout the whole of Ross River Estuary during low spring tides on 16–18 September 2017 and 10 May 2019. Two additional crab species not detected during the 2011 biodiversity survey (Tubuca seismella and Gelasimus vocans) were identified and collected during this second round of sampling and included in morphometric analyses (except those analyses focusing on links between habitat characteristics and morphology, Table 1). All individuals collected in the field were immediately placed on ice for transport to the Estuary and Coastal Wetland Ecosystems Laboratory, James Cook University, Townsville, Australia, where they were frozen at − 20 °C. Subsequently, the crabs were wrapped in 70% formalin-soaked paper towels and sent to the Laboratory for Coastal Ecology and Conservation, Kochi, Japan where they were immediately photographed and digitized as sets of geometric landmarks (see ‘A Standardized Geometric Morphometrics Protocol for Crabs’ section) upon arrival. It was a non-negotiable requirement of Japanese customs to preserve the crabs in formalin. Preservation of crabs, albeit a necessary step, can lead to subtle changes in overall shape (Rufino et al. 2004). Some individuals of Paracleistostoma wardi, G. vocans, Parasesarma messa and Metopograpsus latifrons were damaged during transport on either carapace or claws, preventing their use in geometric morphometric analyses and thus lowering the sample size for these species (Table 1). These species were included in assemblage-level analyses, but no detailed species-specific analyses were conducted due to the small sample size. In addition to the geometric landmarks, we also recorded for each individual the following characteristics: species, sex, wet mass (mg) of the whole crab and of each claw (manus and dactyl) after blotting with tissue paper; carapace depth (mm) measured as the maximum vertical distance between the telson and the top of the carapace; carapace width (mm) at its broadest point; and right eyestalk length (mm).
A Standardized Geometric Morphometrics Protocol for Crabs
A subset of crabs gathered in the field was used to develop and test a new standardised protocol for the collection of landmark sets of crab carapaces and claws for use in geometric morphometric analyses of shape variation within and among species. Standard, widely available photographic equipment was used to allow easy replication of the protocol. Specifically, a Pentax K10D single-lens digital reflex camera with 10.2 effective megapixel CCD sensor was mounted on a tripod, with the camera body facing straight down (i.e. levelled parallel to the table surface on which it was standing) 118 cm above the individual to be photographed (see Online Resource 1). Five focal lengths (24 mm, 50 mm, 70 mm, 100 mm and 200 mm) were tested using Pentax SMC-DA zoom lenses provided with the camera as a kit: 24–55 mm and 50–200 mm (the 50-mm focal length was tested with the 50–200 mm lens). The area photographed was adjusted for the wider focal lengths of 24 mm and 50 mm by raising the crab on a platform of 70 cm and 15 cm height, respectively (Table 2). A low ISO value of 100 was chosen to obtain clearly defined images without grain. Two desk lamps were placed 10 cm to 20 cm on either side of the crab to provide direct lighting (see Online Resource 1). White balance was set manually using the white index of a Kodak Q-13 ‘colour separation guide and grey scale’ card prior to each series of 10 photographs. Light metering was done using spot metering in the centre of the field of view (where the crabs were placed). Focussing was likewise done in the centre of the field of view. Images were taken with a 2-s delay timer and stored in JPEG format. Three experiments were conducted to select the most suitable lens and aperture setting, a fourth experiment analysed error sources.
Experiment 1:
To establish whether the different lenses displace points inward or outward near the edges of the image (horizontal displacement), a sheet of graph paper was photographed at each of the five focal lengths tested, and five replicate measurements of displacement made per focal length. Specifically, a section of 10 subdivisions of graph paper (corresponding to 10 mm of real distance) was selected in the middle of the photograph and this section was set as a scale of 10 mm for the rest of the photograph using the image analysis software ImageJ (Schneider et al. 2012). Subsequently, sections of 10 subdivisions of graph paper were measured at a distance of 1/10th, 1/4th and 1/3rd the width of a photograph, starting from the edge of the photograph. The length of these sections in ImageJ is the photographed distance. Displacement in the photograph could then be identified by taking the ratio of the photographed distance relative to the real distance (i.e. 10 mm). Positive displacement, where the photographed distance is larger than the real distance, indicates barrel distortion. Negative displacement indicates a pincushion effect.
Experiment 2:
A suitable aperture value was selected to ensure that the whole carapace or claw would be in focus while maintaining short exposure times, since longer exposure times increase noise and blur in the image. A 1.5-cm high block of wood marked with an ‘X’ at the top was placed onto a piece of graph paper with millimetre subdivisions. The measure of 1.5 cm was chosen as it is slightly more than the maximum depth of a carapace or claw of the species analysed in this study. It was then checked which apertures between F 5.6 and F 13 gave a sharp image of the ‘X’ and the graph paper.
Experiment 3:
Following the outcomes of the previous two experiments, the degree to which the 200 mm lens displayed two points above each other in three-dimensional space as separate points within a two-dimensional photograph was established (i.e. 3D to 2D displacement). A block of wood with a marking at a height of 1.5 cm on its side was moved from the centre towards the edge of the field of view. Vertical displacement was measured on photographs of the wooden block at 2, 3 and 4 cm from the centre of the field of view (and with graph paper as background). In ImageJ, a scale of 10 mm was then set, which allowed the distance between the bottom of the block and the marked line to be measured on each of these photographs.
Experiment 4:
Once the appropriate photographic setup was established, the final evaluation in protocol development was conducted: assessment of digitization error. The carapace and both claws were photographed and digitized for one male for each of three species with distinct morphologies: Metopograpsus frontalis, Parasesarma longicristatum and Tubuca coarctata. Due to the large difference in size of the claws of male T. coarctata, the digitization error in both major and minor claw landmarks was analysed for this species, while only the right claw was analysed for the other two species. Prior to digitization, carapaces and claws were photographed. Carapaces were prepared for photography by removing the legs and claws, and suspending the carapace horizontally using needle pins attached to 1-cm high wooden blocks. One pin was placed at the front centre between the eyes and two at the bases of the last pair of legs (Online Resource 1). This method of positioning carapaces was found to be precise during pilot work (Online Resources 1 and 2). Individual claws were suspended with a pin at the location where the arm attaches and by placing the manus and pollex of the claw flat on a 1-cm high piece of wood, with the outer surface facing the camera and the inner (palmar) surface facing downward (Online Resource 1). The wooden blocks were covered with graph paper, providing a reference scale for processing the images. Once photographed, images of claws were digitally flipped, if required, so that each appeared as a right claw, thus minimising digitization error due to perceptual differences (Auffray et al. 1996; Klingenberg and McIntyre 1998). Human error during digitization of landmarks from photographs was then assessed using the coefficient of variation (ratio of SD to mean) in carapace and claw centroid size across six repeated landmark measurements per species, representing six times that landmarks were identified and digitized on the same photograph.
Using the newly developed protocol (a stepwise guide can be found in Online Resource 1), all individuals of all species collected throughout Ross River Estuary for geometric morphometrics were each photographed once for the carapace and once for their claws. All carapace and claw images were imported into ImageJ, where a 20-mm section of graph paper was selected and set as a scale for that specific photo. Next, twenty-two landmarks capturing carapace shape, and six landmarks capturing claw shape were recorded. The 22 carapace landmarks were chosen as a set that outlines the main areas of the carapace and are identifiable across species (Online Resource 3). For claws, the 6 landmarks were selected following Rosenberg (2002).
Statistical Analysis
Using generalized Procrustes alignment (GPA), a consensus configuration of geometric landmarks describing the average shape of an organism (e.g. the average individual of a certain crab species or the average crab within an assemblage of species) was obtained for multiple individuals. During this iterative process, landmark sets of individuals were translated to the same origin, rescaled and rotated until the distance between the sets was minimized in Kendall’s shape space (Zelditch et al. 2012). These landmarks were then projected into Euclidean space for further statistical analyses. Further details and mathematical descriptions of geometric morphometric analyses can be found in Bookstein (1991), Rohlf and Marcus (1993) and Marcus et al. (1996).
Species-Specific Analyses
Species-specific analyses were conducted on GPA-aligned landmarks of five species with sufficient sample sizes available (Table 1): three Ocypodoidea: Tubuca coarctata, T. seismella and T. signata, and two Grapsoidea: Metopograpsus frontalis and Parasesarma longicristatum. First, the degree of bilateral symmetry in carapace (left to right side) and claw (left vs. right or minor vs. major claw) shapes was considered. For male Tubuca spp., major vs. minor claws were compared while for females right and left were compared. For M. frontalis, it was noticed that often one claw was slightly larger than the other. The larger claw was then designated as the major and the smaller as the minor claw and bilateral symmetry assessed between major and minor claws. Since there was no apparent difference in claw size for P. longicristatum, the analysis was conducted between right and left claws. Bilateral symmetry was assessed using a two-way Procrustes ANOVA that decomposes shape variation into variation among individuals and among bilateral symmetric sides. The output of this Procrustes ANOVA contains landmark sets that describe the fluctuating asymmetry (the component of shape that deviates from perfect bilateral symmetry) for each individual. A subsequent Procrustes ANOVA on these fluctuating asymmetry landmark sets then assessed the difference in the degree of fluctuating asymmetry between males and females (except for T. coarctata for which only males were available). In these Procrustes ANOVAs, the sum-of-squared Procrustes distances were used as a measure of the sum of squares (Goodall 1991) for which significance was assessed using 1000 randomized permutations.
Secondly, differences in claw sizes between males and females of the same species were considered. The centroid size (mm), determined as the square root of the sum of the squared distances between each landmark and the centroid of the landmark set, was calculated and used as a measure of carapace or claw size. Note that this analysis only considered size differences and that crabs with the same centroid size can have different shapes. The centroid size of major and minor claws was then standardized by dividing them by the centroid size of the carapace of the corresponding individual. A two-way ANOVA subsequently assessed differences in the logarithmic of the standardized claw centroid size between sexes (except for T. coarctata for which only males were available) and between claws (major vs. minor or left vs. right, as defined in the previous paragraph). Additionally, this analysis was repeated for males only, with a one-way ANOVA to assess differences in the logarithmic of the standardized claw size between sides.
A final intraspecific analysis focused on allometric relations between shape and size, accounting for differences among sexes. Specifically, linear regression models were constructed considering the relation between carapace centroid size and each of the following metrics: major (or right) claw centroid size, ratio of major (or right) claw mass to carapace mass, carapace width, carapace depth and right eyestalk length. Additionally, shape relations were established using Procrustes regression (with 1000 randomized permutations to establish significance based on the sum-of-squared Procrustes distances) between (a) carapace shape and carapace size, (b) major (or right hand) claw shape and major (or right hand) claw size and (c) major (or right hand) claw shape and carapace size. Lastly, the degree of morphological integration (i.e. covariance) between carapace shape and major (or right hand) claw shape were established using a two-block partial least squares analysis, where a high correlation suggests a high degree of morphological integration between the two body parts.
Assemblage-Level Analyses
To place the species-specific results into a broader context, we explored the morphological diversity within the broader assemblage and how this might link to the microhabitat the species live in. All eleven species sampled were included in this analysis to generate an overview of the morphological variation at the assemblage level. Nonetheless, the focus of the analyses rested on those species with most replication (i.e. the five species analysed for species-specific patterns) as their position within the assemblage can be most accurately represented.
Assemblage-level variation in either carapace or claw shapes was explored using principal components analysis (PCA) on GPA-aligned landmarks of all individuals of all eleven species collected (Table 1) within the assemblage in tangent space (and plotted in tangent space). The centroid scores of each species on the first and second PC axis were calculated and used in subsequent morphology-environment analyses (RLQ analysis, see below) as numeric values characterizing species based on their shape, relative to other species in the assemblage.
RLQ analysis (Dolédec et al. 1996) was conducted to graphically explore the main patterns of co-variation between the environment (R matrix,) and morphological traits of species (Q matrix), using a species-by-sites matrix (L matrix) as a link. Specifically, relative abundances of the nine species obtained during the biodiversity survey were standardized per site by the total number of individuals caught at that site (i.e. row standardization). The cross-correlation in standardized species relative abundances across sites was then obtained using correspondence analysis (CA). Site weights (i.e. row weights) of the CA were subsequently taken into account when ordinating species versus environmental factors characterizing the nine different habitats identified in the biodiversity survey, using a Hill-Smith ordination (Hill and Smith 1976) to account for mixed categorical and continuous variables used when characterizing habitats. Meanwhile, species weights (i.e. column weights) of the CA were taken into account when ordinating species versus morphological traits using principal components analysis (PCA) to accommodate the continuous variables in the trait dataset. The final RLQ ordination gave scores to the environment at sites, and to the traits of species by maximizing the co-inertia criterion. This criterion is the product of the variance of the environment scores by the variance of the trait scores and by the squared cross-correlation between the environment and trait scores depending on species abundance (Dray et al. 2003). The outputs of the RLQ analysis were used to group species based on their functional similarity using cluster analysis (Kleyer et al. 2012). Specifically, Ward’s hierarchical clustering was performed on the Euclidean distances between species on the first two axes of the RLQ analysis.
Traits considered in the RLQ analysis were the centroid scores of each species on the first and second PC axes of the PCAs displaying assemblage level variation in either carapace (with and without landmarks identifying the position of the eyestalks) as well as minor (or left) and major (or right) claw shapes. Additionally, the length of the right eyestalk and the depth of the carapace (both standardized against the carapace centroid size) were also considered, resulting in a set of ten morphological traits. Environmental factors considered were categorical variables for vegetation and for tidal height, and continuous variables for bank angle and canopy cover.
All analyses were conducted in R version 3.6.0 (R coreTeam 2020), the geomorph package was used for shape analyses (Adams et al. 2019) including residual randomisation permutation procedures (Collyer and Adams 2018, 2019) and the ade4 package for RLQ analyses (Dray and Dufour 2007).
Results
A Standardized Geometric Morphometrics Protocol for Crabs
All five focal lengths tested displayed some degree of barrel effect (inward horizontal displacement of points near the edge of the field of view, Table 2). The 200-mm focal length displayed the smallest barrel effect, which was reduced closer to the centre of the photograph (Table 2). An aperture of F 9 was the smallest aperture that produced sharp images of objects up to 1.5 cm thick with the 200-mm focal length lens. For objects with such thickness, a 3D to 2D displacement of 0.37 mm, 0.43 mm and 0.48 mm occurred at 2 cm, 3 cm and 4 cm from the centre of the image, respectively. Given these results, we opted to use a lens with a 200-mm focal length and keep crabs within the centre third of the photograph.
Digitization error was minimal with the coefficient of variation in centroid size among the six repeated digitisations of landmarks per species and body part being < 0.0032 (Table 3). An exception was the minor claw of Tubuca coarctata, which displayed a higher coefficient of variation of 0.0074. This is likely due to the small size of this claw and subsequent more difficult digitization. Nonetheless, relative to the coefficient of variation among different individuals of the same species, the coefficient of variation due to digitization error was 1 to 2 orders of magnitude smaller (Table 3). A full stepwise protocol for the collection of geometric landmark sets from images of crabs is presented in Online Resource 1.
Species-Specific Results
Symmetry in Carapace and Claws
Most species showed marked deviations from bilateral symmetry in carapace shape, except Tubuca seismella (Table 4). None of the species, however, displayed large differences in the degree of fluctuating asymmetry in carapace shape in relation to sex. Landmarks on the outside of the carapace were generally less variable than those outlining internal regions of the carapace (Fig. 2, Online Resource 4). Variability in landmark position could be linked to difficulties in identifying some of the landmarks, although overall digitization error was low (see ‘A Standardized Geometric Morphometrics Protocol for Crabs’ section). Landmark variability likely mostly reflects intraspecific differences with marked variation in carapace shape among individuals for each of the five species considered (Fig. 2, Table 4).
Selected consensus configurations of carapace and major (right) claws (plots for all species in Supplement 4). Open circles: GPA-aligned landmark sets of individuals; filled circles: consensus landmark set; nr. of ind.: number of individuals analysed per species and body part. For illustration purposes, a grey shaded area is drawn for Metopograpsus frontalis, to visualise landmark positions relative to full carapace or claw. (claw is upside down with movable finger towards bottom of graph)
Regarding claw shape, all five species showed deviations from bilateral symmetry (Table 4). Only T. seismella, however, showed a large difference in the degree of fluctuating asymmetry in claw shape in relation to sex (Table 4). The inflection point at the bottom of the claw was more variable, especially in the X-direction, in Parasesarma longicristatum and Metopograpsus frontalis (Fig. 2), while there was no obvious difference in the degree of variability in position among the other claw landmarks.
Regarding claw centroid size (standardized against carapace centroid size), there were differences between sexes for all species for which males and females were available (Table 5), with distinct differences between the size of both claws of male Tubuca spp. (Table 5). For all species, there was less than 11% difference between left and right standardized claw centroid sizes in females. For males, this difference was less than 13% in M. frontalis and P. longicristatum. Meanwhile, in T. seismella and Tubuca signata, the standardized centroid size of the major claw was 2.7 and 3.7, respectively, times larger than the minor claw, and 2.8 and 3.7, respectively, times larger than the major claw of females. In M. frontalis and P. longicristatum, the largest claw in males was about 15% and 34%, respectively, larger than in females.
Allometry in Size and Shapes
Tubuca signata was the smallest and M. frontalis the largest of the five species analysed (Table 5). The major (or right) claw centroid size showed a positive relationship with the carapace centroid size (mean R2: 0.92, minimum: 0.79, maximum: 0.97 across all species, Fig. 3, Online Resource 6), which was influenced by sex, with a less steep slope in female T. signata, and to a lesser extent also in female M. frontalis and P. longicristatum.
Selected allometric relations between carapace centroid size (mm) and different size measurements for species containing sufficient females (black) and males (grey) for analysis (plots for all species in Supplement 6). Mass ratio: major (or right) claw mass/carapace mass
The carapace centroid size increased with increasing carapace width, with average R2 values of linear models (considering sex and its interaction with carapace width) of 0.93 (minimum: 0.85, maximum: 0.99, Fig. 3, Online Resource 6) across all five species. A similar, albeit less strong, relationship was observed with carapace depth (mean R2: 0.82, minimum: 0.50, maximum: 0.96 across species, Fig. 3, Online Resource 6). Neither sex nor the interaction between sex and carapace width had an effect, except for P. longicristatum and M. frontalis where the relationship with carapace width and depth, respectively, depended on sex (Fig. 3). A relationship between carapace centroid size and eyestalk length was observed for some species, specifically M. frontalis, P. longicristatum and T. coarctata (Fig. 3). Models were also developed for claw mass, but none of the species displayed a strong relationship between carapace centroid size and claw mass, although for P. longicristatum and M. frontalis the relationship was dependent on sex (Fig. 3).
Carapace shape and claw shape showed high degrees of morphological integration as evidenced by high correlations (average among the five species 0.78 ± 0.11 SD) between the two body parts. Additionally, carapace shape and the major (or right) claw shape changed with carapace centroid size in P. longicristatum, T. coarctata and T. signata, with an effect and interaction of sex on the relationship for T. signata (Figs. 4 and 5, Online Resource 6). Tubuca seismella also showed a relationship between carapace shape and centroid size, but the relationship between major claw shape and carapace size was weak (Fig. 5, Online Resource 6).
Procrustes regression between carapace centroid size (mm) and shape. Deformation grids left and right of plots show variation in shape from smallest to largest centroid size respectively. Regression score on the y-axis captures carapace shape of one individual in one value relative to consensus configuration among individuals for a given species
Procrustes regression between carapace centroid size (mm) and major (right) claw shape. Deformation grids left and right of plots show variation in shape from smallest to largest centroid size respectively. Regression score on y-axis captures shape of one individual in one value relative to consensus configuration among individuals for a given species
Assemblage-Level Results
A PCA on carapace shapes, used as an exploration of assemblage-level variation in morphology that could be uncovered using the new protocol, distinguished three groups: the three Tubuca spp. (analysed previously for species-specific patterns) and G. vocans on one end of the first PC axis, Grapsoidea (including M. frontalis and P. longicristatum, analysed previously for species-specific patterns) on the other end, and a group consisting of the Ocypodoidea, Australoplax tridentata and Paracleistostoma wardi in between (Fig. 6). The distinction between the taxonomic groups along the 1st and 2nd PC axes, capturing 54.5% and 12.4% of shape variation, respectively, was driven to a large extent by the landmarks indicating the positioning of the eyestalks. However, even after excluding eyestalk landmarks from the shape analyses, the results indicated a separation between the three taxonomic groups. The 1st and 2nd PC axes captured 24.0% and 22.4% respectively in this re-analysis, which suggested a relative lengthening of the carapace and an accompanying increase in the space occupied by the gastric region in the Grapsoidea, as well as a change in shape of the cardiac region (Fig. 6).
PCA of carapace shape variation in tangent space; each point represents one individual. Top panel: results including all landmarks; bottom panel: results excluding landmarks indicating eyestalk position. Deformation grids display shape changes from top left to the bottom right (i.e. from minimum 1st PC and maximum 2nd PC to maximum 1st PC and minimum 2nd PC). Deformation grids are not to scale relative to each other. Individuals are enclosed in coloured polygons per species (for G. vocans, the two individuals available are connected with a line)
Most variation in claw shapes was captured along the first PC axes for both minor and major (or left and right) claws (Fig. 7). Even though the distinction between claw shapes was less evident than for carapace shape, Ocypodoidea were located more on one side of the plot, characterized by elongated claws. Meanwhile, Grapsoidea were located on the other side with shorter, sturdier claws. An exception was the Ocypodoidea A. tridentata which appeared to be more similar in shape for both claws to Grapsoidea (however, only six and four specimens were available to characterise the major and minor claw, respectively, of this species).
PCA of claw shape variation in tangent space. Each point represents one individual. Top panel: major (right) claws; Bottom panel: minor (left) claws. Deformation grids display changes in shape from top left to bottom right (i.e. from minimum 1st PC and maximum 2nd PC to maximum 1st PC and minimum 2nd PC values). Deformation grids are not to scale relative to each other. Individuals are enclosed in coloured polygons per species (for G. vocans, the two individuals available are connected with a line)
Habitat Preference—Morphology Linkage
Nine species of intertidal crabs across two superfamilies and five families were recorded in the biodiversity survey (Table 1). Austroplax tridentata and T. signata were the most prevalent species, making up an average of 24.7 ± 7.0% SD and 22.1 ± 3.5% SD, respectively, of the numbers of individuals recorded at the four locations , followed by T. coarctata and P. longicristatum with means of 14.2 ± 2.2% SD and 11.5 ± 3.9% SD, respectively. All other species, on average, each made up < 10% of the total number of individuals caught.
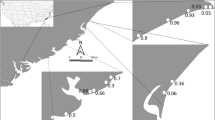
The RLQ analyses captured 65.7% and 31.3% of the co-variation between morphological traits of crabs and the habitat characteristics of the intertidal estuarine landscape they inhabited along the first and second ordination axis respectively. The main objective of this analysis in the context of our research was to investigate whether the novel protocol could be used to explore such morphology-environment linkages. Ward’s hierarchical clustering of the Euclidean distances between species on the first two axes of the RLQ analysis revealed four groups (Fig. 8). A first group, T. coarctata and T. signata, was associated with bare habitats, irrespective of tidal height or inclination (Figs. 8 and 9). A second group, consisting of M. frontalis and Metopograpsus latifrons was associated with steep angled, low intertidal banks (without clear preference for the dominant vegetation bordering these banks). The third group, consisting of A. tridentata and P. wardi was associated with higher intertidal habitats containing Sporobolus spp. or Ceriops spp. vegetation. The final group clustered Parasesarma messa and Parasesarma erythrodactylum in habitats characterized by Ceriops spp. (and to some extend Rhizophora stylosa) vegetation and increasing canopy cover, largely unrelated to tidal height. P. longicristatum was also clustered in this fourth group, although its abundances increased more towards lower tidal habitats with steep angles.
Left panel: Hill Smith ordination of species versus habitat characteristics (grid cell unit: 1, vectors of habitat characteristics are plotted twice as long for clarity). Right panel: RLQ ordination of morphological traits (black arrows) versus habitat characteristics (black triangles indicate endpoints of habitat characteristic vectors) considering species abundances at sites as weights (grid cell unit: 0.5). Deformation grids show minimum and maximum configurations along 1st axes of assemblage level PCAs (Fig. 6 and 7). Carapace–eye: carapace configurations without eyestalk landmarks
Due to the different morphological traits present within species in each of the four groups, the RLQ analysis was able to associate different habitat preferences with distinct morphological traits (Fig. 9). For instance, steep angled, low intertidal banks dominated by R. mangle or Ceriops spp. were inhabited by crabs characterised by sturdier claws (both left and right claw), a relatively flat body and relatively longer carapaces with short eyestalks located on the sides. Examples are species of the families Sesarmidae and Grapsidae. Meanwhile, crabs with deeper bodies and long eyestalks, such as Tubuca spp., are more abundant on sparsely vegetated habitats higher in the intertidal zone.
Discussion
A Standardized Geometric Morphometrics Protocol for Crabs
Geometric morphometric analyses have been applied to intertidal crabs (e.g. Rosenberg 2002; Hopkins and Thurman 2010; Hampton et al. 2014), but a standardized protocol for the collection and assessment of data has not been described, so we developed a standardized protocol for the collection of geometric landmark sets of crab carapace and claw shapes (Online Resource 1) and applied it to the analysis of morphological variation within and among crab species in intertidal mangrove habitats. Specimens were 6.4–33.4 mm carapace width at their broadest point. The size range of crabs (and other organisms) examined could potentially be expanded, subject to further validation, by using a zoom lens for the photography or by increasing the distance between the organism and the camera. Crabs in the present study had relatively flat carapaces and claws, suitable for two-dimensional imaging. This allowed the detection of a number of ecologically informative intra- and interspecific shape relations for intertidal crabs consistent with two-dimensional geometric morphometric analyses of freshwater insects (Orlofske and Baird 2014).
Interpretation of observed shape changes needs to take into account the three-dimension to two-dimension conversion when photographs are taken (Cardini 2014). For instance, a relative shift of the cardiac region towards the back of the carapace was observed with increasing carapace centroid size in Tubuca spp. (Fig. 4). This was potentially caused by the posterior of the carapace becoming more curved in larger crabs, and should thus be considered when interpreting results. Hopkins and Thurman (2010), in their analysis of morphological variation of Tubuca spp. using geometric morphometrics, included a landmark indicating the position of the dorso-lateral margin (Online Resource 3). They suggested that shifts in the position of lateral margins could further assist in interpreting the degree of carapace curvature in three dimensions (Hopkins and Thurman 2010). Since this landmark could not be reliably identified among all species in the current study, it was not included. Alternatively, the shape of the cardiac region of Tubuca spp. also changed with size, potentially hinting at a true physiological change. Technologies for three-dimensional landmark collection such as laser scanning, tomography and photogrammetry offer interesting perspectives for further detailed analyses including for organisms with very different body sizes or anatomies (e.g. Márquez and Averbuj 2017; Semple et al. 2019). Nonetheless, our protocol for two-dimensional data collection used readily available, low-cost technology amenable to many research, conservation and monitoring programs.
Small morphological variations, particularly in the degree of fluctuating asymmetry, have been suggested as a sensitive early warning sign of environmental stress (Hoffmann et al. 2005). The detection and analyses of such variations require precise positioning and digitization to ensure that the variability observed is linked to the individuals themselves, and not to positioning or digitization errors. Both error sources were minimal in the protocol developed in the current study (Table 3). Individuals damaged during transport (predominantly through squashing) were excluded from morphometric image collection, which reduced the sample size for Paracleistostoma wardi, Gelasimus vocans, Parasesarma messa and Metopograpsus latifrons. No species-specific analyses were conducted on these species. Therefore, results regarding their assemblage-level position are more uncertain than for the other species where more individuals were available to define their general shape. It should also be noted that the preservation in formalin for transport could have weakened the structural integrity of the carapace and claws of some specimens, resulting in slight shape changes. This could potentially explain the lower correlation between carapace centroid size and carapace depth compared to the carapace width in some specimens (Fig. 3, Online Resource 6). Careful consideration should be given to this potential source of error in future studies. An alternative explanation to the different correlation strengths is that the carapace depth might inherently be a more variable measure because carapace and telson can move relative to each other. In fact, generally, carapace width at its broadest point is used as an indicator of crab size (e.g. Freire et al. 1996; Seed and Hughes 1997; Silva et al. 2017).
Allometry and Sexual Variation
Fiddler crabs (Subfamily: Gelasiminae) have traditionally been studied for their sexual dimorphism, with males having one enlarged claw, and related social behaviour (Valiela et al. 1974; Oliveira and Custodi 1998; Allen and Levinton 2007). Our results confirmed differences in the claw centroid size (standardized against carapace centroid size) between sexes among all four species for which males and females were available (Table 5). These included two fiddler crabs in the genus Tubuca (T. seismella and T. signata) and also two Grapsids (M. frontalis and Parasesarma longicristatum). These results suggest that sexual dimorphism in claw size occurs among intertidal mangrove crabs in different taxonomic groups. However, while all males of the three Tubuca spp. investigated (T. signata, T. seismella and T. coarctata, Table 5) showed a strong difference in size between major and minor claws. There was no difference in claw size for M. frontalis and P. longicristatum. Hence, although there are differences between males and females, there appears to be a difference in the degree of expression of sexual dimorphism in males among species. Metopograpsus frontalis and P. longicristatum occur in visually complex environments where visual signals are more restricted in range as compared to the open habitats where Tubuca spp. occur (Fig. 9, Vermeiren and Sheaves 2015a, b). Subject to validation across more intertidal crab species, this fuels the hypothesis that sexual selection is a weaker secondary selective force on visual morphological traits (such as claw size) for species living in complex environments.
No obvious difference between sexes in the degree of fluctuating asymmetry of carapace shape was observed for any of the five species (Table 4). Nonetheless, many species displayed individual variation and fluctuating asymmetry in carapace as well as claw shape (Table 4). Consequently, other factors, in addition to sexual differences, influenced morphological variation within species including, among others, allometric relationships and environmental factors (Parsons 1992; Voje 2016). Diet, for instance, can have a strong influence on claw morphology. For example, the intertidal crab Pachygrapsus marmoratus when living on exposed rocky shores with a high abundance of hard-shelled prey items (mussels) possessed larger claws than the same species feeding on easier to crush barnacles on sheltered rocky shores (Silva et al. 2009). Likewise, heterochely in relation to diet is common among portunid crabs, which often have a large, strong crusher claw and a smaller cutter claw (e.g. Seed and Hughes 1997).
In the current study, both claw and carapace shapes changed with a high degree of correlation (morphological integration) among them, indicating that body parts do not grow independently of each other. Additionally, claw shape also changed with carapace centroid size (Fig. 5). Such allometric shape changes can affect the performance of the morphological trait. For example, changes in claw shapes with body size resulted in a negative correlation between the mechanical advantage of claws and body size (expressed as carapace width) for the portunid crabs Necora puber, Liocarcinus depurator and Liocarcinus navigator (Freire et al. 1996). Moreover, L. navigator also displayed a negative correlation with muscle ratio, indicating that claws grow more in size than in muscle (Freire et al. 1996). Hence, a larger claw is not necessarily a stronger claw. In this case, the changing strength of the claws corresponded with ontogenetic diet shifts for these species (Freire et al. 1996). In the current study, allometric relations such as the carapace centroid size vs. claw centroid size varied between sexes for all species (M. frontalis, P. longicristatum and T. signata) analysed for male-female allometric relationships (Fig. 3). These results indicate a degree of sexual variation in allometric relations within representatives of different taxonomic groups. Sexual selection and other influences such as diet are unlikely to operate in isolation. In fact, the development of a larger claw (e.g. for mate attraction) likely comes at a fitness disadvantage, for example as a trade off in mechanical advantage and thereby potentially available prey items (Freire et al. 1996) or as an increased visibility towards predators (Koga et al. 2001).
A strong allometric relationship between carapace centroid size and carapace width at its broadest point (Fig. 3, Online Resource 6) confirms that carapace width can be used as a quick measure of crab size (e.g. Freire et al. 1996; Seed and Hughes 1997; Silva et al. 2009). Meanwhile, the generally poorer relationship between carapace centroid size and mass likely reflects discrete growth associated with moulting stages where the exoskeleton grows incrementally while mass increases continuously between moults (Brylawski and Miller 2006). This also implies that crab weight is a more reliable indicator of growth and body condition than measurements of the exoskeleton dimensions.
Habitat Characteristics—Morphology Linkages
Morphological traits showed considerable variation among species and an apparent linkage with the environment. The shape of the carapace showed taxonomic groupings (Fig. 6), which could indicate a certain degree of phylogenetic constraint so closely related species occupy more similar niches and display more similar trait characteristics than would be expected by simple Brownian evolutionary motion (Losos 2008). In agreement with this hypothesis, the Hill Smith ordination (Fig. 9) of species versus habitat characteristics and Ward’s hierarchical clustering of this ordination (Fig. 8) showed taxonomically related species clustering in relation to distinct habitat characteristics. By contrast, the greater overlap and more continuous gradient in claw shapes among species (Fig. 7), compared to the fairly distinct grouping of carapace shapes, could indicate a stronger role of secondary factors such as diet, sexual variation or environmental factors to influence claw shapes, and thus less of a phylogenetic signal. These analyses explored the potential of the novel protocol as a basis for further in-depth study. Analysis of a wider range of species, coupled with genetic analyses, could provide further insights into the degree to which carapace and claw shapes are preserved phylogenetically.
The current study explored associations between habitat preferences and distinct morphologies of crab species. Eyestalk length and their positioning on the carapace linked with occurrence of species on bare habitats (longer eyestalks) or on banks with steep angles and habitats with structurally complex R. stylosa vegetation (eyestalks towards the sides of the carapace). This is in agreement with known insights into eye morphology where the eyes on long stalks provide a good telescopic view of the environment, suitable for open habitats, while shorter eyestalks towards extreme ends of the carapace provide better stereoscopic views suitable for living in environments where gauging distances is crucial (e.g. root structures or steep banks, Zeil et al. 1986; Zeil and Hemmi 2006). Other morphological changes can likely be traced to the available diet in the habitats and the trophic position of the crabs. For instance, sturdier claws of Metopograpsus spp. might reflect the more omnivorous diet and higher trophic position (including opportunistic carnivory) compared to the elongated claws of Tubuca spp. which often feed on microphytobenthos and scoop up mud rather than crush prey (Vermeiren et al. 2015). Freire et al. (1996) identified that the portunid L. navigator, with a diet composed mainly of sea weeds and soft organisms, had less mobile chela with lower muscle ratios compared to the portunid L. depurator which consumed more hard-shelled prey. Vermeiren et al. (2015) studying spatial variation in diet preferences among intertidal mangrove crabs identified that M. frontalis and Metopograpsus latifrons displayed a more opportunistic diet preference, able to shift among locations, compared to the specialized diet of T. coarctata and T. seismella. However, whether this diet opportunism among locations is also evidenced in spatial variation in claw morphologies is not yet known. Likewise, to what extent morphological variations are driven by phenotypic plasticity or an adaptive response with a genetic basis remains to be determined.
Conclusion
The current study applied a novel protocol to test hypotheses regarding the relative influence of sexual, allometric and habitat-related influences on morphologies of crab species within an intertidal estuarine assemblage. The study complements work on crab morphology that focus on specific factors or species. Results indicated that morphological trait variability is driven by multiple, interacting factors. For example, many allometric relations differed among sexes (Fig. 3). Likewise, there was a close relationship between habitat preferences and morphological traits, yet various factors such as sexual variation, allometric relations, diet and phylogeny all had some influence. Consequently, future studies should take an integrative, multi-factor approach when considering morphological trait variation. The standardised protocol developed and validated in the current study provides a sensitive tool to approach such analyses.
References
Adams, D.C., M.L. Collyer, and A. Kaliontzopoulou. 2019. Geomorph: software for geometric morphometric analyses. R package version 3.1.0. https://cran.r-project.org/package=geomorph.
Allen, B.J., and J.S. Levinton. 2007. Costs of bearing a sexually selected ornamental weapon in fiddler crabs. Functional Ecology 21: 154–161.
Auffray, J.C., P. Alibert, S. Renaud, A. Orth, and F. Bonhomme. 1996. Fluctuating asymmetry in Mus musculus subspecific hybridization: Traditional and Procrustes comparative approach. In Advances in morphometrics, ed. L.F. Marcus, M. Corti, A. Loy, G.J.P. Naylor, and D.E. Slice, 275–283. New York: Plenum Press.
Bookstein, F.L. 1991. Morphometric tools for landmark data. Cambridge: Cambridge University Press.
Bookstein, F.L., B.L. Chernoff, R.L. Elder, J.M. Humphries Jr., G.R. Smith, and R.E. Strauss. 1985. Morphometrics in evolutionary biology. Philadelphia: The Academy of Natural Sciences Philadelphia.
Bosire, J.O., J.G. Kairo, J. Kazungu, N. Koedam, and F. Dahdouh-Guebas. 2005. Predation on propagules regulates regeneration in a high-density reforested mangrove plantation. Marine Ecology Progress Series 299: 149–155.
Botto, F., and O. Iribarne. 1999. Effect of the burrowing crab Chasmagnathus granulata (Dana) on the benthic community of a SW Atlantic coastal lagoon. Journal of Experimental Marine Biology and Ecology 241 (2): 263–284.
Botto, F., G. Palomo, O. Iribarne, and M.M. Martinez. 2000. The effect of southwestern Atlantic burrowing crabs on habitat use and foraging activity of migratory shorebirds. Estuaries 23 (2): 208–215.
Breuker, C.J., J.S. Patterson, and C.P. Klingenberg. 2006. A single basis for developmental buffering of Drosophila wing shape. PLoS One 1 (1): e7.
Brylawski, B.J., and T.J. Miller. 2006. Temperature-dependent growth of the blue crab (Callinectes sapidus): A molt process approach. Canadian Journal of Fisheries and Aquatic Sciences 63 (6): 1298–1308.
Campbell, G.R. 1977. A comparative study of the distribution, physiology, morphology, and behaviour of five Sesarminae species occurring along the Ross River estuary. Townsville: James Cook University.
Cardini, A. 2014. Missing the third dimension in geometric morphometrics: how to assess if 2D images really are a good proxy for 3D structures? Hystrix, the Italian Journal of Mammalogy 25: 73–81.
Castiglioni, D.S., and M.L. Negreiros-Fransozo. 2006. Reproductive cycle of the fiddler crab Uca rapax (Smith) (Crustacea, Brachyura, Ocypodidae) at a degraded estuary in Paraty, Rio de Janeiro, Brazil. Revista Brasileira de Zoologia 23 (2): 331–339.
Collyer, M.L., and D.C. Adams. 2018. RRPP: An R package for fitting linear models to high-dimensional data using residual randomization. Methods in Ecology and Evolution 9 (7): 1772–1779.
Collyer, M.L., and D.C. Adams. 2019. RRPP: linear model evaluation with randomized residuals in a permutation procedure, version 0.6.0. https://cran.r-project.org/web/packages/RRPP.
Dolédec, S., D. Chessel, C.J.F. ter Braak, and S. Champely. 1996. Matching species traits to environmental variables: a new three-table ordination method. Environmental and Ecological Statistics 3 (2): 143–166.
Dray, S., and A.B. Dufour. 2007. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22: 1–20.
Dray, S., D. Chessel, and J. Thioulouse. 2003. Co-inertia analysis and the linking of ecological data tables. Ecology 84 (11): 3078–3089.
Dye, A.H., and T.A. Lasiak. 1986. Microbenthos, meiobenthos and fiddler crabs: trophic interactions in a tropical mangrove sediment. Marine Ecology Progress Series 32: 259–264.
Escarpa, M., O. Iribarne, and D. Navarro. 2004. Effects of the intertidal burrowing crab Chasmagnathus granulatus on infuanal zonation patterns, tidal behavior, and risk of mortality. Estuaries 27 (1): 120–131.
Freire, J., M.P. Sampedro, and E. González-Gurriarán. 1996. Influence of morphometry and biomechanics on diet selection in three portunid crabs. Marine Ecology Progress Series 137: 111–121.
Fruciano, C. 2016. Measurement error in geometric morphometrics. Development Genes and Evolution 226: 139–158.
Frusher, S.D. 1988. Key to crabs associated with mangroves in North Queensland. Townsville: Australian Institute of Marine Science press.
Goodall, C.R. 1991. Procrustes methods in the statistical analysis of shape. Journal of the Royal Statistical Society: Series B 53: 285–339.
Hampton, K.R., M.J. Hopkins, J.C. McNamara, and C.L. Thurman. 2014. Intraspecific variation in carapace morphology among fiddler crabs (Genus Uca) from the Atlantic coast of Brazil. Aquatic Biology 20 (1): 53–67.
Hill, M.O., and A.J.E. Smith. 1976. Principal component analysis of taxonomic data with multi-state discrete characters. Taxon 25 (2-3): 249–255.
Hoffmann, A.A., R.E. Woods, E. Collins, K. Wallin, A. White, and J.A. McKenzie. 2005. Wing shape versus asymmetry as an indicator of changing environmental conditions in insects. Australian Journal of Entomology 44 (3): 233–243.
Hopkins, M.J., and C.L. Thurman. 2010. The geographic structure of morphological variation in eight species of fiddler crabs (Ocypodidae: genus Uca ) from the eastern United States and Mexico. Biological Journal of the Linnean Society 100 (1): 248–270.
Horton, T., et al. 2020. World register of marine species. Available from https://www.marinespecies.org at VLIZ. Accessed 2020-06-01.
How, M.J., J.M. Hemmi, J. Zeil, and R. Peters. 2008. Claw waving display changes with receiver distance in fiddler crabs, Uca perplexa. Animal Behaviour 75 (3): 1015–1022.
Kleyer, M., S. Dray, F. Bello, J. Leps, R.J. Pakeman, B. Strauss, W. Thuiller, and S. Lavorel. 2012. Assessing species and community functional responses to environmental gradients: Which multivariate methods? Journal of Vegetation Science 23 (5): 805–821.
Klingenberg, C.P. 2009. Morphometric integration and modularity in configurations of landmarks: tools for evaluating a priori hypotheses. Evolution & Development 11: 405–421.
Klingenberg, C.P., and G.S. McIntyre. 1998. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 52: 1363–1375.
Koch, V., and M. Wolff. 2002. Energy budget and ecological role of mangrove epibenthos in the Caeté estuary, North Brazil. Marine Ecology Progress Series 228: 119–130.
Koga, T., P.R.Y. Backwell, J.H. Christy, M. Murai, and E. Kasuya. 2001. Male-biased predation of a fiddler crab. Animal Behaviour 62 (2): 201–207.
Lee, S.Y. 2008. Mangrove macrobenthos: Assemblages, services, and linkages. Journal of Sea Research 59 (1-2): 16–29.
Losos, J.B. 2008. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecology Letters 11 (10): 995–1003.
Macintosh, D.J., E.C. Ashton, and S. Havanon. 2002. Mangrove rehabilitation and intertidal biodiversity: a study in the Ranong mangrove ecosystem, Thailand. Estuarine, Coastal and Shelf Science 55 (3): 331–345.
Marcus, L.F., M. Corti, A. Loy, G.J.P. Naylor, and D.E. Slice. 1996. Advances in morphometrics. New York: Plenum Press.
Márquez, F., and A. Averbuj. 2017. Sexual dimorphism in the shell of a nassariid gastropod. A 3D geometric morphometrics approach. Journal of the Marine Biological Association of the United Kingdom 97 (2): 249–255.
Nagelkerken, I., S.J.M. Blaber, S. Bouillon, P. Green, M. Haywood, L.G. Kirton, J.O. Meynecke, J. Pawlik, H.M. Penrose, A. Sasekumar, and P.J. Somerfield. 2008. The habitat function of mangroves for terrestrial and marine fauna: a review. Aquatic Botany 89 (2): 155–185.
Oliveira, R.F., and M.R. Custódio. 1998. Claw size, waving display and female choice in the European fiddler crab, Uca tangeri. Ethology Ecology and Evolution 10 (3): 241–251.
Orlofske, J.M., and D.J. Baird. 2014. Incorporating continuous trait variation into biomonitoring assessments by measuring and assigning trait values to individuals or taxa. Freshwater Biology 59 (3): 477–490.
Parsons, P.A. 1992. Fluctuating asymmetry: a biological monitor of environmental and genomic stress. Heredity 68: 361–364.
Poore, G.C. 2004. Marine decapod Crustacea of southern Australia: A guide to identification. Melbourne: CSIRO publishing.
R core Team. 2020. R: A language and environment for statistical computing. Vienna Austria: R foundation for statistical computing, version 3.6.3 https://www.R-project.org/.
Rohlf, F.J., and L.F. Marcus. 1993. A revolution in morphometrics. Trends in Ecology & Evolution 8 (4): 129–132.
Rosenberg, M.S. 2002. Fiddler crab claw shape variation: a geometric morphometric analysis across the genus Uca (Crustacea: Brachyura: Ocypodidae ). Biological Journal of the Linnean Society 75: 147–162.
Rufino, M., P. Abelló, and A.B. Yule. 2004. The effect of alcohol and freezing preservation on carapace size and shape in Liocarcinus depurator (Crustacea, Brachyura). In Morphometrics Applications in Biology and Paleontology, ed. A.M.T. Elewa, 45–53. Berlin Heidelberg: Springer-Verlag.
Schneider, C.A., W.S. Rasband, and K. Eliceiri. 2012. NIH image to ImageJ: 25 years of image analysis. Nature Methods 9 (7): 671–675.
Seed, R., and R.N. Hughes. 1997. Chelal characteristics and foraging behaviour of the blue crab Callinectes sapidus Rathbun. Estuarine, Coastal and Shelf Science 44 (2): 221–229.
Semple, T.L., R. Peakall, and N.J. Tatarnic. 2019. A comprehensive and user-friendly framework for 3D-data visualisation in invertebrates and other organisms. Journal of Morphology 280 (2): 223–231.
Sheaves, M., R. Johnston, and R.M. Connolly. 2010. Temporal dynamics of fish assemblages of natural and artifical tropical estuaries. Marine Ecology Progress Series 410: 143–157.
Silva, A.C.F., S. Brazão, S.J. Hawkins, R.C. Thompson, and D.M. Boaventura. 2009. Abundance, population structure and claw morphology of the semi-terrestrial crab Pachygrapsus marmoratus (Fabricius, 1787) on shores of differing wave exposure. Marine Biology 156 (12): 2591–2599.
Silva, A.C.F., M. Shapouri, R. Cereja, A. Dissanayake, and C. Vinagre. 2017. Variations in crab claw morphology and diet across contrasting inter-tidal habitats. Marine Ecology 38: 1–8.
Valiela, I., D.F. Babiec, W. Atherton, S. Seitzinger, and C. Krebs. 1974. Some consequences of sexual dimorphism: Feeding in male and female fiddler crabs, Uca pugnax (Smith). The Biological Bulletin 147 (3): 652–660.
Vermeiren, P. 2013. A landscape level understanding of habitat associations to integrate intertidal crabs into ecosystem models of tropical estuaries. PhD thesis, James Cook University, Townsville.
Vermeiren, P., and M. Sheaves. 2014. A remote photographic technique for high replication, large scale understanding of spatial distribution patterns of intertidal crabs. Hydrobiologia 724 (1): 79–89.
Vermeiren, P., and M. Sheaves. 2015a. Predictable habitat associations of four crab species across the low intertidal landscape of a tropical estuary over time. Estuaries and Coasts 38 (1): 285–295.
Vermeiren, P., and M. Sheaves. 2015b. Modeling intertidal crab distribution patterns using photographic mapping among tropical Australian estuaries. Estuaries and Coasts 38 (5): 1545–1556.
Vermeiren, P., K. Abrantes, and M. Sheaves. 2015. Generalist and specialist feeding crabs maintain discrete trophic niches within and among estuarine locations. Estuaries and Coasts 38 (6): 2070–2082.
Voje, K.L. 2016. Scaling of morphological characters across trait type, sex, and environment a meta-analysis of static allometries. The American Naturalist 187 (1): 89–98.
von Hagen, H. 1993. Waving display in females of Uca polita and of other Australian fiddler crabs. Ethology 93: 3–20.
Werry, J., and S.Y. Lee. 2005. Graspid carbs mediate link between mangrove litter production and estuarine planktonic food chains. Marine Ecology Progress Series 293: 165–176.
Wolff, M., V. Koch, and V. Isaac. 2000. A trophic flow model of the Caeté mangrove estuary (North Brazil) with considerations for the sustainable use of its resources. Estuarine, Coastal and Shelf Science 50: 786–803.
Zeil, J., and J.M. Hemmi. 2006. The visual ecology of fiddler crabs. Journal of Comparative Physiology 192 (1): 1–25.
Zeil, J., G. Nalbach, and H.O. Nalbach. 1986. Eyes, eye stalks and the visual world of semi-terrestrial crabs. Journal of Comparative Physiology 159 (6): 801–811.
Zelditch, M.L., D.L. Swiderski, and H.D. Sheets. 2012. Chapter 4: theory of shape. In Geometric Morphometrics for Biologists, a Primer, 2nd ed. Cambridge: Academic Press.
Acknowledgements
We acknowledge the College of Arts, Society and Education, James Cook University, for use of their photo studio.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Judy Grassle
Electronic Supplementary Material
ESM 1
(PDF 699 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vermeiren, P., Lennard, C. & Trave, C. Habitat, Sexual and Allometric Influences on Morphological Traits of Intertidal Crabs. Estuaries and Coasts 44, 1344–1362 (2021). https://doi.org/10.1007/s12237-020-00856-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-020-00856-4