Abstract
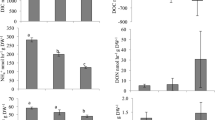
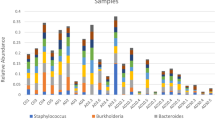
Aquaculture currently provides half of all fish for human consumption, and this proportion is expected to increase to meet the growing global demand for protein. As aquaculture, including oyster farming, expands, it is increasingly important to understand effects on coastal ecosystems. The broad-scale ecological effects of oyster aquaculture are well documented; however, less is known regarding the influence of oyster aquaculture on sediment bacterial communities. To better understand this relationship, we compared three different oyster farming practices that varied in oyster biomass and proximity of oysters to the sediment. We used high-throughput sequencing and quantitative polymerase chain reaction to examine the effect of oyster farming on sediment bacterial communities. We examined the entire bacterial community and looked specifically at bacteria that support essential estuarine ecosystem services (denitrifiers), as well as bacteria that can be detrimental to human health (members of the Vibrio genus). We found that oyster biomass increased Vibrio richness and sediment carbon content, which influenced bacterial community composition. When compared to reference sites, the overall abundance of bacteria was increased by the bottom planting method, but the associated increases in denitrifiers and Vibrio were not significant. We were unable to detect V. parahaemolyticus, V. vulnificus, or V. cholera, the three most common Vibrio pathogens, in any sample, suggesting that oyster farming did not enhance these potential human pathogens in sediments at the time of sampling. These results highlight how differences in oyster farming practice can affect sediment bacterial communities, and the ecosystem services they provide.






Similar content being viewed by others
References
Albakosh, M.A., R.K. Naidoo, B. Kirby, and R. Bauer. 2015. Identification of epiphytic bacterial communities associated with the brown alga Splachnidium rugosum. Journal of Applied Phycology 28: 1891–1901. doi:10.1007/s10811-015-0725-z.
Anderson, M.J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46.
Austin, B. 2010. Vibrios as causal agents of zoonoses. Veterinary Microbiology 140: 310–317.
Biddle, J.F., S. Fitz-Gibbon, S.C. Schuster, J.E. Brenchley, and C.H. House. 2008. Metagenomic signatures of the Peru margin subseafloor biosphere show a genetically distinct environment. Proceedings of the National Academy of Sciences 105: 10583–10588.
Borrego, J.J., D. Castro, A. Luque, and C. Paillard. 1996. Vibrio tapetis sp. nov., the causative agent of the brown ring disease affecting cultured clams. International Journal of Systematic and Evolutionary Microbiology 46: 480–484.
Bowen, J.L., B.B. Ward, H.G. Morrison, J.E. Hobbie, I. Valiela, L.A. Deegan, and M.L. Sogin. 2011. Microbial community composition in sediments resists perturbation by nutrient enrichment. The ISME Journal 5: 1540–1548. doi:10.1038/ismej.2011.22.
Bowen, J.L., A.R. Babbin, P.J. Kearns, and B.B. Ward. 2014. Connecting the dots: linking nitrogen cycle gene expression to nitrogen fluxes in marine sediment mesocosms. Frontiers in Microbiology 5: 429. doi:10.3389/fmicb.2014.00429.
Caffrey, J.M., J.T. Hollibaugh, and B. Mortazavi. 2016. Living oysters and their shells as sites of nitrification and denitrification. Marine Pollution Bulletin 112 (1–2): 86–90.
Caporaso, J.G., J. Kuczynski, J. Stombaugh, K. Bittinger, F.D. Bushman, E.K. Costello, N. Fierer, A.G. Peña, J.K. Goodrich, J.I. Gordon, G.A. Huttley, S.T. Kelley, D. Knights, J.E. Koenig, R.E. Ley, C.A. Lozupone, D. McDonald, B.D. Muegge, M. Pirrung, J. Reeder, J.R. Sevinsky, P.J. Turnbaugh, W.A. Walters, J. Widmann, T. Yatsunenko, J. Zaneveld, and R. Knight. 2010. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7: 335–336.
Caporaso, J.G., C.L. Lauber, W.A. Walters, D. Berg-Lyons, J. Huntley, N. Fierer, S.M. Owens, J. Betley, L. Fraser, M. Bauer, N. Gormley, J.A. Gilbert, G. Smith, and R. Knight. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal 6: 1621–1624.
Cerco, C.F., and M.R. Noel. 2007. Can oyster restoration reverse cultural eutrophication in Chesapeake Bay? Estuaries and Coasts 30: 331–343.
Chase, E., S. Young, and V.J. Harwood. 2015. Sediment and vegetation as reservoirs of Vibrio vulnificus in the Tampa Bay estuary and Gulf of Mexico. Applied and Environmental Microbiology 81: 2489–2494. doi:10.1128/aem.03243-14.
Coen, L.D., M.W. Luckenbach, and D.L. Breitburg. 1999. The role of oyster reefs as essential fish habitat: a review of current knowledge and some new perspectives. In Fish habitat: essential fish habitat and rehabilitation, ed. L.R. Benaka, 22nd ed., 438–454. Bethesda: American Fisheries Society.
Coen, L.D., R.D. Brumbaugh, D. Bushek, R.E. Grizzle, M.W. Luckenbach, M.H. Posey, S.P. Powers, and S.G. Tolley. 2007. Ecosystem services related to oyster restoration. Marine Ecology Progress Series 341: 303–307. doi:10.3354/meps34130.
Coenye, T., and P. Vandamme. 2003. Intragenomic heterogeneity between multiple 16S ribosomal RNA operons in sequenced bacterial genomes. FEMS Microbiology Letters 228: 45–49.
Cole, K.M., J. Supan, A. Ramirez, and C.N. Johnson. 2015. Suspension of oysters reduces the populations of Vibrio parahaemolyticus and Vibrio vulnificus. Letters in Applied Microbiology 61: 209–213. doi:10.1111/lam.12449.
DeSantis, T.Z., P. Hugenholtz, N. Larsen, M. Rojas, E.L. Brodie, K. Keller, T. Huber, D. Dalevi, P. Hu, and G.L. Andersen. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology 72: 5069–5072.
Edgar, R.C. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461. doi:10.1093/bioinformatics/btq461.
Edgar, R.C., B.J. Haas, J.C. Clemente, C. Quince, and R. Knight. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200.
Eren, A.M., L. Maignien, W.J. Sul, L.G. Murphy, S.L. Grim, H.G. Morrison, and M.L. Sogin. 2013. Oligotyping: differentiating between closely related microbial taxa using 16S rRNA gene data. Method in Ecology and Evolution 4: 1111–1119.
Falkowski, P.G., T. Fenchel, and E.F. Delong. 2008. The microbial engines that drive Earth’s biogeochemical cycles. Science 320: 1034–1039. doi:10.1126/science.1153213.
FAO. 2014. The state of world fisheries and aquaculture 2014. Rome: Food and Agiculture Organization of United Nations.
Garrido-Maestu, A., M.J. Chapela, E. Penaranda, J.M. Vieites, and A.G. Cabado. 2014. In-house validation of novel multiplex real-time PCR gene combination for the simultaneous detection of the main human pathogenic vibrios (Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus). Food Control 37: 371–379.
Grizzle, R.E., J.K. Greene, M.W. Luckenbach, and L.D. Coen. 2006. A new in situ method for measuring seston uptake by suspension-feeding bivalve molluscs. Journal of Shellfish Research 25: 643–649.
Haskin, H.H., L.A. Stauber, and J.A. Mackin. 1966. Minchinia nelsoni n. sp. (Haplosporida, Haplosporidiidae): causative agent of the Delaware Bay oyster epizootic. Science 153: 1414–1416. doi:10.1126/science.153.3742.1414.
Haven, D.S., and R. Morales-Alamo. 1966. Aspects of biodeposition by oysters and other invertebrate filter feeders. Limnology and Oceanography 11: 487–498.
Higgins, C.B., K. Stephenson, and B.L. Brown. 2011. Nutrient bioassimilation capacity of aquacultured oysters: quantification of an ecosystem service. Journal of Environment Quality 40: 271–277. doi:10.2134/jeq2010.0203.
Hoellein, T.J., and C.B. Zarnoch. 2014. Effect of eastern oysters (Crassostrea virginica) on sediment carbon and nitrogen dynamics in an urban estuary. Ecological Applications 24: 271–286.
Hoellein, T.J., C.B. Zarnoch, and R.E. Grizzle. 2015. Eastern oyster (Crassostrea virginica) filtration, biodeposition, and sediment nitrogen cycling at two oyster reefs with contrasting water quality in Great Bay estuary (New Hampshire, USA). Biogeochemistry 122: 113–129.
Howarth, R.W. 2008. Coastal nitrogen pollution: a review of sources and trends globally and regionally. Harmful Algae 8: 14–20.
Iwamoto, M., T. Ayers, B.E. Mahon, and D.L. Swerdlow. 2010. Epidemiology of seafood-associated infections in the United States. Clinical Microbiology Reviews 23: 399–411.
Jayakumar, A., S.W.A Naqvi, and B.B. Ward. 2009. Distribution and relative quantification of key genes involved in fixed nitrogen loss from the Arabian Sea oxygen minimum zone. In, eds. J.D. Wiggert, R.R. Hood, S.W.A. Naqvi, K.H. Brink, and S.L. Smith, Indian Ocean Biogeochemical Processes and Ecological Variability, 187–203. Washington DC: American Geophysical Union.
Johnson, C.N., J.C. Bowers, K.J. Griffitt, R.W. Clostio, S. Pei, E. Laws, R.N. Paranjpye, M.S. Strom, A. Chen, N.A. Hasan, A. Huq, N.F. Noriea, D.J. Grimes, and R.R. Colwell. 2012. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States). Applied and Environmental Microbiology 78: 7249–7257.
Jordan, S.J. 1987. Sedimentation and remineralization associated with biodeposition by the American oyster Crassostrea virginica (Gmelin), PhD Dissertation Thesis, University of Maryland, College Park.
Joye, S.B., and I.C. Anderson. 2008. Nitrogen cycling in coastal sediments. In Nitrogen in the Marine Environment, eds. D. Capone, D. Bronk, E. Carpenter, and M. Mulhollond, 867–915. doi:10.1016/b978-0-12-372522-6.00019-0.
Kearns, P.J., J.H. Angell, S.G. Feinman, and J.L. Bowen. 2015. Long-term nutrient addition differentially alters community composition and diversity of genes that control nitrous oxide flux from salt marsh sediments. Estuarine, Coastal and Shelf Science 154: 39–47.
Kellogg, M.L., J.C. Cornwell, M.S. Owens, and K.T. Paynter. 2013. Denitrification and nutrient assimilation on a restored oyster reef. Marine Ecology Progress Series 480: 1–19.
Lawson, N. et al. 2011. The state of Duxbury Bay, 2009. Duxbury, MA: Duxbury Bay Management Commission, 17 pp.
Lefcheck, J.S. 2015. piecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods in Ecology and Evolution 7: 573–579.
Lindemann, S., C.B. Zarnoch, and D. Castignetti. 2016. Effect of eastern oysters (Crassostrea virginica) and seasonality on nitrite reductase gene abundance (nirS, nirK, nrfA) in an urban estuary. Estuaries and Coasts 39: 218–232. doi:10.1007/s12237-015-9989-4.
Lozupone, C.A., M.E. Lladser, D. Knights, J. Stombaugh, and R. Knight. 2011. UniFrac: an effective distance metric for microbial community comparison. The ISME Journal 5: 169–172.
Mackin, J.G., and A. Collier. 1950. Preliminary note on the occurrence of a new protistan parasite, Dermocystidium marinum n. sp. in Crassostrea virginica (Gmelin). Science 111: 328–329. doi:10.1126/science.111.2883.328.
Mann, R., M. Southworth, J.M. Harding, and J.A. Wesson. 2009. Population studies of the native eastern oyster, Crassostrea virginica, (Gmelin, 1791) in the James River, Virginia, USA. Journal of Shellfish Research 28: 193–220. doi:10.2983/035.028.020.
Mansergh, S., and J.P. Zehr. 2014. Vibrio diversity and dynamics in the Monterey Bay upwelling region. Frontiers in Microbiology 5: 48. doi:10.3389/fmicb.2014.00048.
Massachusetts Department of Marine Fisheries. 2014. Massachusetts Marine Fisheries 2014 Annual Report. Boston: Department of Fish and Game, Commonwealth of Massachusetts.
Newell, R.I.E., J.C. Cornwell, and M.S. Owens. 2002. Influence of simulated bivalve biodeposition and microphytobenthos on sediment nitrogen dynamics: a laboratory study. Limnology and Oceanography 47: 1367–1379. doi:10.4319/lo.2002.47.5.1367.
Newell, R.I.E., T.R. Fisher, R.R. Holyoke, and J.C. Cornwell. 2005. Influence of eastern oysters on nitrogen and phosphorus regeneration in Chesapeake Bay, USA. In, eds. R. Dame and S. Olenin, The Comparitive Roles of Suspension Feeders in Ecosystems, 93–120. Dordrecht, The Netherlands: Springer.
Newton, A., M. Kendall, D.J. Vugia, O.L. Henao, and B.E. Mahon. 2012. Increasing rates of vibriosis in the United States, 1996–2010: review of surveillance data from 2 systems. Clinical Infectious Diseases 54: S391–S395. doi:10.1093/cid/cis243.
Peterson, C.H., J.H. Grabowski, and S.P. Powers. 2003. Estimated enhancement of fish production resulting from restoring oyster reef habitat: quantitative valuation. Marine Ecology Progress Series 264: 249–264.
Piehler, M.F., and A.R. Smyth. 2011. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2: 1–17.
Powell, E.N., R. Mann, Y. Kim, and D. Bushek. 2015. The allometry of oysters: spatial and temporal variation in the length–biomass relationships for Crassostrea virginica. Journal of the Marine Biological Association of the United Kingdom 96: 1127–1144. doi:10.1017/s0025315415000703.
R Core Team. 2014. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Sawabe, T., I. Sugimura, M. Ohtsuka, K. Nakano, K. Tajima, Y. Ezura, and R. Christen. 1998. Vibrio halioticoli sp. nov., a non-motile alginolytic marine bacterium isolated from the gut of the abalone Haliotis discus hannai. International Journal of Systematic and Evolutionary Microbiology 48: 573–580.
Sawabe, T., Y. Fujimura, K. Niwa, and H. Aono. 2007. Vibrio comitans sp. nov., Vibrio rarus sp. nov. and Vibrio inusitatus sp. nov., from the gut of the abalones Haliotis discus discus, H. gigantea, H. madaka and H. rufescens. International Journal of Systematic and Evolutionary Microbiology 57: 916–922.
Scallan, E., R.M. Hoekstra, F.J. Angulo, R.V. Tauxa, M.A. Widdowson, S.L. Roy, J.L. Jones, and P.M. Griffin. 2011. Foodborne illness acquired in the United States—major pathogens. Emerging Infectious Diseases 17: 7–15.
Shaw, W.N. 1962. Raft culture of oysters in Massachusetts. Fishery Bulletin 61: 481–495.
Shaw, W.N. 1963. Comparison of growth of four strains of oysters raised in Taylors pond, Chatham, Mass. Fishery Bulletin 63: 11–17.
Shumway, S.E. 1996. Natural environmental factors. In The eastern oyster: Crassostrea virginica, ed. V.S. Kennedy and A.F. Eble, 467–513. College Park: Maryland Sea Grant.
Smyth, A.R., N.R. Geraldi, and M.F. Piehler. 2013. Oyster-mediated benthic-pelagic coupling modifies nitrogen pools and processes. Marine Ecology Progress Series 493: 23–30. doi:10.3354/meps10516.
Smyth, A.R., M.F. Piehler, and J.H. Grabowski. 2015. Habitat context influences nitrogen removal by restored oyster reefs. Journal of Applied Ecology 52: 716–725.
Southworth, M., J.M. Harding, J.A. Wesson, and R. Mann. 2010. Oyster (Crassostrea virginica, Gmelin 1791) population dynamics on public reefs in the great Wicomico River, Virginia, USA. Journal of Shellfish Research 29: 271–290. doi:10.2983/035.029.0202.
Thomas, A.D., D. Stamatis, J. Bertsch, M. Isbandi, J. Jansson, J.K. Mallajosyuta, I. Pagani, E.A. Lobos, and N.C. Kyrpides. 2014. The Genomes OnLine Database (GOLD) v. 5: a metadata management system based on a four level (meta) genome project classification. Nucleic Acids Research 43: D1099. doi:10.1093/nar/gku950.
Thompson, F.L., T. Iida, and J. Swings. 2004a. Biodiversity of vibrios. Microbiology and Molecular Biology Reviews 68: 403–431.
Thompson, J.R., M.A. Randa, A. Tomita-Mitchell, E. Lim, and M.F. Polz. 2004b. Diversity and dynamics of a North Atlantic coastal Vibrio community. Applied and Environmental Microbiology 70: 4103–4110.
Wang, Q., G.M. Garrity, J.M. Tiedje, and J.R. Cole. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology 73: 5261–5267.
World Bank. 2013. Fish to 2030: Prospects for Fisheries and Aquaculture. 83177. Washington D.C.: Agricultural and Environmental Services Discussion Paper 03.
Zumft, W.G. 1997. Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews 61: 533–616.
Acknowledgements
We would like to thank John Brawley and Island Creek Oyster Company for allowing us to sample their oyster leases and for help and guidance in designing this experiment. We would like to thank Michael Tlusty for connecting us with the Island Creek team. We would also like to acknowledge the guidance of Jarrett Byrnes in statistical analyses and sample design. Thank you to Patrick Kearns, John Angell, and all the members of the Bowen Lab for assistance. Sequence analyses were made possible through the use of the supercomputing facilities managed by the Research Computing Department at the University of Massachusetts Boston. Sediment characteristics were analyzed at the Environmental Analytical Facility at the University of Massachusetts Boston. Funding for this project was provided by the University of Massachusetts Boston Doctoral Dissertation Research Grant (SGF), the Sanofi Genzyme Educational Foundation Grant (YRF), and National Science Foundation Research Experience for Undergraduates awards DBI-1062374 and DBI-1359242 to Dr. Alan Christian and Dr. Robin Hannigan. Finally, we would like to acknowledge the constructive comments of external reviewers that improved the quality of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marianne Holmer
Electronic supplementary material
ESM 1
(DOCX 391 kb)
Rights and permissions
About this article
Cite this article
Feinman, S.G., Farah, Y.R., Bauer, J.M. et al. The Influence of Oyster Farming on Sediment Bacterial Communities. Estuaries and Coasts 41, 800–814 (2018). https://doi.org/10.1007/s12237-017-0301-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0301-7