Abstract
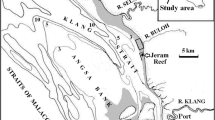
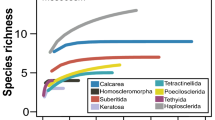
The polychaete (sabellariid-spionid) reefs at Jeram shore (Malaysia) grow up on soft-bottom mudflats and appear short-lived. It is postulated that such reef building results from the succession of polychaete species in response to the changing environment modulated by the extreme hydrometeorological events. To elucidate the biological succession of the reef cycle in relation to the environment, two reef patches on the intertidal mudflat were studied, both spatially (horizontal and vertical community structure) and temporally (June 2012 to January 2014). The Jeram polychaete reef cycles through four phases within a year: pre-settlement phase (March–May), growth phase comprising primary (May–November) and secondary (October–January) successional stages, stagnation phase (December–January), and destruction phase (January–March). The reef dynamics appear to be linked to the regional monsoon climate and local hydrological conditions. At the onset of the southwest monsoon (May), strong erosive forces initiate the reef’s primary succession of the growth phase where the dominant polychaete Sabellaria jeramae colonize and rapidly grow on the exposed lag deposits of shells. During the northeast monsoon (November–March), stronger depositional forces cover the developed reef with fine sediments that are colonized by another polychaete, the spionid Polydora cavitensis during the reef’s secondary succession of the growth phase. On the muddy substrate surrounding the reef clumps, mudflat polychaetes were the most abundant macrobenthos followed by anomurans, gastropods, carideans, and brachyurans. However, these mudflat macrobenthos play no obvious or direct role in initiating the growth of the reef which is likely the result of settlement of dispersed polychaete larvae from unknown offshore reefs. On the other hand, the reef presence has a positive effect on the presence or abundance of surrounding mudflat macrobenthos such as mudflat polychaetes, shrimps, crabs, and gastropods.





Similar content being viewed by others
References
Almaca, C. 1990. Structure and interactions in the crab community inhabiting sabelariid worm colonies at Praia de Ribeira D'Ilhas (Ericeira, Portugal). Arq Museu Bocage. N.S. 1(37): 505–519.
Bellan, G., G. Desrosiers, and A. Willsie. 1988. Use of an annelid pollution index for monitoring a moderately polluted littoral zone. Marine Pollution Bulletin 19: 662–665.
Blake, J.A. 1996. Family Spionidae Grube, 1850. In In: Taxonomic atlas of the benthic fauna of the Santa Maria Basin and western Santa Barbara Channel. Vol. 6. The Annelida. Part 3, ed. J.A. Blake et al., 81–224. California: Santa Barbara Mus Nat Hist.
Blake, J.A., and J.D. Evans. 1973. Polydora and related genera (Polychaeta: Spionidae) as borers in mollusk shells and other calcareous substrates. The Veliger 15: 235–249.
Bremec, C., C. Carcedo, M.C. Piccolo, E.D. Santos, and S. Fiori. 2012. Sabellaria nanella (Sabellariidae): from solitary subtidal to intertidal reef-building worm at Monte Hermoso, Argentina (39°S, south-west Atlantic). Journal of the Marine Biological Association of the United Kingdom: 1–6.
Buchanan, J.B. 1984. Sediment analysis. In Methods for the study of marine benthos, ed. N.A. Holme and A.D. McIntyre, 41–65. Oxford: Blackwell.
Chong, V.C., A. Sasekumar, and E. Wolanski. 1996. The role of mangroves in retaining penaeid prawn larvae in Klang Strait, Malaysia. Mangroves and Salt Marshes 1: 11–22.
Curtis, L.A. 1978. Aspects of the population dynamics of the polychaete Sabellaria vulgaris Verrill, in the Delaware Bay. Estuaries 1: 73–84.
Day, J.H. 1967. A monograph on the Polychaeta of Southern Africa. Part I Errantia and Part II Sedentaria. British Museum (Natural History), London.
De Smet, B., L. Godet, J. Fournier, N. Desroy, M. Jaffré, M. Vincx, and M. Rabaut. 2013. Feeding grounds for waders in the bay of the Mont saint-Michel (France): the Lanice conchilega reef serves as an oasis in the tidal flats. Marine Biology 160: 751–761.
DID. 2009. Malaysian coastal inventory (Chapter 5), DID Manual, Vol. 3: Coastal management, page 5A–12, Drainage and Irrigation Department, Government of Malaysia, Kuala Lumpur, Malaysia.
Dubois, S., C. Retière, and F. Olivier. 2002. Biodiversity associated with Sabellaria alveolata (Polychaeta: Sabellariidae) reefs: effects of human disturbances. Journal of the Marine Biological Association of the UK 82: 817–826.
Dubois, S., J.A. Commito, F. Olivier, and C. Retière. 2006. Effects of epibionts on Sabellaria alveolata (L.) biogenic reefs and their associated fauna in the bay of Mont Saint-Michel. Estuarine, Coastal and Shelf Science 68: 635–646.
Fauchald, K. 1977. The polychaete worms. Definitions and keys to the orders, families and genera. Natural History Museum of Los Angeles County. Science Series 28: 1–188.
Fausto-Filho, J., and E. Furtado. 1970. Nota preliminar sobre a fauna das colônias de Sabellariidae do litoral do Estado do Ceará (Annelida, Sedentaria). Revista Brasileira de Biologia 30: 285–289.
Fitri, A., R. Hashim, K.I. Song, and S. Motamedi. 2015. Evaluation of morphodynamic changes in the vicinity of low-crested breakwater on cohesive shore of Carey island, Malaysia. Coastal Engineering Journal 57(4): 1–27.
Friedrichs, M., G. Graf, and B. Springer. 2000. Skimming flow induced over a simulated polychaete tube lawn at low population densities. Marine Ecology Progress Series 192: 219–228.
Giangrande, A. 1997. Polychaete reproductive patterns, life cycles and life histories: an overview oceanography and marine biology: an annual review 35: 323–386.
Gore, R.H., L.E. Scotto, and L.J. Becker. 1978. Community composition, stability, and trophic partitioning in decapod crustaceans inhabiting some subtropical Sabellariid worm reefs studies on decapod crustacea from the Indian River region of Florida. IV. Bulletin of Marine Science 28: 221–248.
Gruet, Y. 1970. Faune associée des “récifs”édifiés par l’Annélide Sabellaria alveolata (Linné) en baie du Mont Saint-Michel: banc des Hermelles. Memoires de la Société des Sciences de Cherbourg 54: 1–21.
Gruet, Y. 1971. Morphologie, croissance et faune associée des récifs de Sabellaria alveolata (Linné) de la Bernerie-en-Retz (Loire Atlantique). Tethys 3: 321–380.
Gruet, Y. 1982. Recherches sur l'écologie des récifs d'Hermelles édifiés par l'Annélide Polychéte Sabellaria alveolata (Linné). PhD thesis, Université des Sciences et Techniques, Nantes, France.
Gruet, Y. 1986. Spatio-temporal changes of Sabellarian reefs built by the sedentary polychaete Sabellaria alveolata (Linné). Marine Ecology 7: 303–319.
Haig, J. 1960. The Porcellanidae (crustacea Anomura) of the eastern Pacific. Allan Hancock Pacific Expedition 24: 1–440.
Hayward, B.W., and J.D. Stilwell. 1995. Floating cockle shells (Austrovenus stutchburyi)—their significance to paleoenvironmental assessments. Tane 35: 143–148.
Heuers, J. 1998. A model on the distribution and abundance of the tube building polychaete Lanice conchilega (Pallas, 1766) in the intertidal of the Wadden Sea. Verh. Ges. Ökol. 28: 207–215.
Holme, N.A., and A.D. Mclntyre. 1971. IBP handbook no 16. Methods for the study of marine benthos. Oxford: Blackwell.
Johnson, D. 1958. The Indo-West Pacific species of the genus Polyonyx (Crustacea, Decapoda, Porcellanidae). Annals of Zoology (Agra) 2: 95–118.
Jost, L. 2006. Entropy and diversity. Oikos 113: 363–375.
La Porta, B., and L. Nicoletti. 2009. Sabellaria alveolata (Linnaeus) reefs in the central Tyrrhenian Sea (Italy) and associated polychaete fauna. Zoosymposia 2: 527–536.
Lomônaco, C., A.S. Santos, and M.L. Christoffersen. 2011. Effects of local hydrodynamic regime on the individual’s size in intertidal Sabellaria (Annelida: Polychaeta: Sabellariidae) and associated fauna at Cabo Branco beach, north-East Brazil. Marine Biodiversity Records 4: e76.
Main, M.B., and W.G. Nelson. 1988. Tolerance of the sabellariid polychaete Phragmatopoma lapidosa Kinberg to burial, turbidity and hydrogen sulfide. Marine Environmental Research 26: 39–55.
Martin, D., and T.A. Britayev. 1998. Symbiotic polychaetes: review of known species. Oceanogr Marine Biology 36: 217–340.
McCarthy, D.A., C.M. Young, and R.H. Emson. 2003. Influence of wave-induced disturbance on seasonal spawning patterns in the sabellariid polychaete Phragmatopoma lapidosa. Marine Ecology Progress Series 256: 123–133.
Miyake, S. 1943. Studies on the crab-shaped Anomura of Nippon and adjacent waters. J Dept Agric, Kyushu Imp Univ 7(3): 49–158.
National Hydrographic Centre. 2002. Tide tables Malaysia. Lumut: Royal Malaysian Navy.
Nelson, W.G., and M.B. Main. 1985. Criteria for beach nourishment: biological guidelines for sabellariid worm reef, Florida Sea Grant College Program. Technical Paper 33.
Ng, P.K.L., and A. Sasekumar. 1993. A new species of Polyonyx Stimpson, 1858, of the P. sinensis group (Crustacea: Decapoda: Anomura: Porcellanidae) commensal with a chaetopterid worm from peninsular Malaysia. Zoologische mededelingen 67: 467–472.
Nishi, E., K. Matsuo, M. Capa, S. Tomioka, H. Kajihara, E.K. Kupriyanova, and G. Polgar. 2015. Sabellaria jeramae, a new species (Annelida: Polychaeta: Sabellariidae) from the shallow waters of Malaysia, with a note on the ecological traits of reefs. Zootaxa 4052: 555–568.
Noernberg, M.A., J. Fournier, S. Dubois, and J. Populus. 2010. Using airborne laser altimetry to estimate Sabellaria alveolata (Polychaeta: Sabellariidae) reefs volume in tidal flat environments. Estuarine, Coastal and Shelf Science 90: 93–102.
Palma, A., and F.P. Ojeda. 2002. Abundance, distribution and feeding patterns of a temperate reef fish in subtidal environments of the Chilean coast: the importance of understory algal turf. Revista Chilena de Historia Natural 75: 189–200.
Pawlik, J.R. 1988a. Larval settlement and metamorphosis of sabellariid polychaetes, with special reference to Phragmatopoma lapidosa, a reef-building species, and Sabellaria floridensis, a non-gregarious species. Bulletin of Marine Science 43: 41–60.
Pawlik, J.R. 1988b. Larval settlement and metamorphosis of two gregarious sabellariid polychaetes: Sabellaria alveolata compared with Phragmatopoma californica. Journal of the Marine Biological Association of the UK 68: 101–124.
Pearson, T.H., and R. Rosenberg. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr Mar Biol Ann Rev 16: 299–311.
Polgar, G., E. Nishi, I. Idris, and C.J. Glasby. 2015. Tropical polychaete community and reef dynamics: insights from a Malayan Sabellaria (Annelida: Sabellariidae) reef. Raffles Bulletin of Zoology 63: 401–417.
Posey, M.H., A.M. Pregnall, and R.A. Graham. 1984. A brief description of a subtidal sabellariid (Polychaeta) reef on the southern Oregon coast. Pacific Science 38: 28–33.
Qian, P.Y., D. Rittschor, B. Sreedhar, and F. Chia. 1999. Macrofouling in unidirectional flow: miniature pipes as experimental models for studying the effects of hydrodynamics on invertebrate larval settlement. Marine Ecology Progress Series 191: 141–151.
Rabaut, M., K. Guilini, G. Van Hoey, M. Vincx, and S. Degraer. 2007. A bio-engineered soft-bottom environment: the impact of Lanice conchilega on the benthic species-specific densities and community structure. Estuarine, Coastal and Shelf Science 75: 525–536.
Rabaut, M., M. Vincx, and S. Degraer. 2009. Do Lanice conchilega (sandmason) aggregations classify as reefs? Quantifying habitat modifying effects. Helgoland Marine Research 63: 37–46.
Ribero, L., and G. Polgar. 2012. The polychaete reefs of Jeram, Selangor. In Mangrove and coastal environment of Selangor, Malaysia, ed. A. Sasekumar and V.C. Chong, 87–95. Kuala Lumpur: University of Malaya Press.
Rivosecchi, T. 1961. Observazioni sulle biocenosi del banco a Sabellaria di Lavino. Estr. Rend. Accad. Nazionale XL 4: 1–11.
Sankolli, K., and S. Shenoy. 1965. On the occurrence of the tube-worm Loimia medusa (Savigny) in Bombay waters and its commensalism with a porcellanid crab. Journal of the Bombay Natural History Society 62: 316–320.
Sato-Okoshi, W. 2000. Polydorid species (Polychaeta: Spionidae) in Japan, with descriptions of morphology, ecology and burrow structure. 2. Non-boring species. Journal of the Marine Biological Association of the UK 80: 443–456.
Seilacher, A. 1984. The Jeram model: event condensation in a modern intertidal environment. In Sedimentary and evolutionary cycles, 336–341: Springer.
Sloan, N.J.B., and E. Irlandi. 2008. Burial tolerances of reef-building Sabellariid worms from the east coast of Florida. Estuarine, Coastal and Shelf Science 77: 337–344.
Taylor, P.R., and M.M. Littler. 1982. The roles of compensatory mortality, physical disturbance, and substrate retention in the development and organization of a sand-influenced, rocky-intertidal community. Ecology: 135–146.
Theede, H., A. Ponat, K. Hiroki, and C. Schlieper. 1969. Studies on the resistance of marine bottom invertebrates to oxygen-deficiency and hydrogen sulphide. Marine Biology 2: 325–337.
Watson, R.L. 1971. Origin of shell beaches, Padre Island, Texas. Journal of Sedimentary Research 41: 1105–1111.
Wells, H.W. 1970. Sabellaria reef masses in Delaware Bay. Chesapeake Science 11: 258–260.
Wilson, D.P. 1971. Sabellaria colonies at Duckpool, North Cornwall, 1961–1970. Journal of the Marine Biological Association of the United Kingdom 51: 509–580.
Woodin, S.A. 1978. Refuges, disturbance, and community structure: a marine soft-bottom example. Ecology: 274–284.
Acknowledgments
The authors would like to thank the University of Malaya Research Grant (UMRG) RG131-11SUS for financial support. This work forms part of JJE’s MSc thesis (in preparation). Dr. Khang Tsung Fei (Institute of Mathematical Sciences, University of Malaya) advised JJE on data presentation aspect, drew attention to Jost’s effective number of species, and read the manuscript. Special thanks to Dr. Chris Glasby from the Museum and Art Gallery of the Northern Territory, Australia, and Dr. Stanislas Dubois from the Muséum National d’Histoire Naturelle, France, for identification of polychaetes. Prof. Peter Ng Kee Lin of the Department of Biological Science, National University of Singapore, is acknowledged for identification of brachyurans, anomurans, and carideans. Thanks are due to the Malaysian Meteorological Department for providing wind data. We are grateful to Dr. G. Polgar and an anonymous reviewer for their useful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Patricia Ramey-Balci
Electronic supplementary material
Online Resource 1
(DOC 1382 kb)
Online Resource 2
(DOC 41 kb)
Online Resource 3
(DOC 242 kb)
Online Resource 4
(DOC 112 kb)
Appendices
Appendix 1
Appendix 2
Rights and permissions
About this article
Cite this article
Eeo, J.J., Chong, V.C. & Sasekumar, A. Cyclical Events in the Life and Death of an Ephemeral Polychaete Reef on a Tropical Mudflat. Estuaries and Coasts 40, 1418–1436 (2017). https://doi.org/10.1007/s12237-017-0217-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0217-2