Abstract
Taxonomic data is essential to advance the discovery and description of biodiversity, as well as the study of evolutionary processes. Emerging large-scale datasets and new methods of analysis have provided different approaches to describe biodiversity. Here, we present a review of the taxonomic history in Cycadales including an analysis of historical taxonomic concepts and approaches used for species delimitation. We examine the trends in the publication of new species following taxonomic works in books, journals and horticultural catalogues, monographic projects and floras where species treatments were published. In addition, we review the studies concerning species delimitations using the literature available in scientific journals appearing in the database ISI Web of Knowledge. The approaches used were discussed throughout all research focused on empirical and theoretical considerations in each study. We review the current state of the studies on causal processes that have given rise to the currently recognized diversity. The trend shows that taxonomic work on discovery and description of species has been intensive in the last 40 years culminating in 38.8% of binomials published. As a result, we consider the relevance of the monographs and floras for identification of species for other biological disciplines and the content of these contributions is compared and discussed. A total of six criteria (diagnosability, phenetic, phylogenetic, genotypic cluster, niche specialization and coalescent) were detected from the following three approaches to species delimitation within Cycadales: traditional, integrative taxonomy, and monophyletic. In all cases, the results from these species delimitations not only provided a taxonomic treatment or proposed a new species, but also supposedly clarified the other species involved as a result of the new taxonomic concept of the new species described. Most investigations of species delimitation used the traditional approach or a phenetic criteria. Finally, we discuss evolutionary studies on causal processes involved in cycad diversity. This is considered in the context of species delimitation as hypothesis testing for a successful evaluation of variation in both genetic and morphological understanding.
Similar content being viewed by others
Introduction
Scientific names are vital for accessing scientific information in all fields of biology. Over the centuries, the names have provided not only an effective means of communication and information transfer, but also conceptual and explanatory properties (Knapp, 2000; Valdecasas et al., 2014; Wheeler, 2023). However, there is a controversy concerning the actual existence of species (Slater, 2016; Mishler, 2021). There is an extensive literature on this subject (Dohzhansky, 1935; Cronquist, 1978; Zachos, 2016a, b, c; Sigwart, 2019; Wheeler, 2023; Wilkins, 2023). We are not discussing these arguments here because we have to use what has been used historically. We do suggest that Crowson (1970) provides an excellent summary of the types of species concepts that have been used historically. A name is a hypothesis applied to an evolutionary entity. Although the names are key resources for understanding global diversity, they are one of the greatest challenges in systematics (Knapp et al., 2004; Wheeler, 2023). Each name implies a taxonomic concept, which contains multiple sources of evidence associated that constitute the classified biological entity, such as geographic distribution, habitat, characters, among others (Franz et al., 2008). The taxonomic concepts are the result of a species concept applied to the circumscription of a species by an author and their meaning, both the species and the concept, can change through the time (Mallet & Willmott, 2003; Knapp et al., 2004; Wheeler, 2004). The different meanings that a same name acquires are due to redefinitions arising from new evidence and/or different interpretations by either the same author or others. This cumulative information produces a partial disconnection between the binomial and its conceptual definitions (Franz et al., 2008).
Monographs are comprehensive taxonomic treatments that systematize and synthesize the phenotypic and genotypic complexity of the organisms included (Grace et al., 2021). In these systematic treatments, the species hypotheses are tested, and the history of the taxonomic concepts of the family, genus and species are condensed (Marhold et al., 2013). A monograph is vital for the identification of species and the discovery of new taxa (Grace et al., 2021; Wheeler, 2023). Currently, new analytical tools have been developed and applied in systematics that have enhanced the discovery of new species (Wheeler et al., 2012; Zhang, 2020). However, monographs and taxonomic reviews have become neglected, which has resulted in either the over- or underestimation of diversity in several groups. The mismatched relationship between advances in the use of molecular evidence for taxonomic purposes and the limited development of monographs is now common. Under integrative approaches, monographs would provide key tools in studying the evolutionary history of species, the causes underlying phenotypic diversity, as well as developmental mechanisms and conservation (Grace et al., 2021; Wheeler, 2023).
Species delimitation is the process of identifying biological units through the recognition of evolutionary patterns (Carstens et al., 2013). In the delimitation of species, different methods are used to study population patterns, and propose classification systems (Sites & Marshall, 2003). These can be classified in non-tree and tree-based methods, where different approaches are involved with each having their own conceptual implications (Sites & Marshall, 2004). The traditional approach is based on strategies and methods historically applied in species delimitation and has been largely attributed to traditional morphological taxonomy (Sites & Marshall, 2003). The approach “integrative taxonomy” uses multiple sources of evidence and methods to infer boundaries between species by considering life history, phenology, reproduction, morphology, genetic diversity, ecological niche, and geographic distribution patterns (DeSalle et al., 2005; Goldstein & DeSalle, 2011). Because species are dynamic entities under different selective pressures, one of the main challenges of this approach is the integration of the results from these different methods and sources.
The limits within species complexes are particularly difficult. The study of these groups of species has been aided by new methods in the era of genomics (Knowles & Carstens, 2007). In the last decade, there has been a considerable increase in research aimed at inferring the boundaries between species in many plant groups (Prata et al., 2018). However, the synthesis of information and the recognition of taxonomic concepts are necessary to take advantage of the potential that these new methods can offer in our understanding of biological diversity and how it developed historically. Some efforts have recently emerged to integrate this taxonomic knowledge with new data through modern botanical monographs and highlighting the importance of these works, which are more pressing due to biodiversity loss (Marhold et al., 2013; Muñoz-Rodríguez et al., 2019). For most plant groups, the monographs date back more than a century, and groups with high species diversity have not been completely monographed (Grace et al., 2021). This lack shows the difficulty of making more efficient use of new data to propose robust species hypotheses, even in the cases with relatively low species diversity such as in Cycadales.
Cycadales, known as cycads, are an ancient group of gymnosperms with a high extinction risk (Brenner et al., 2003; Donaldson, 2003). Considering the unique morphological characters of cycads and their phylogenetic position as the earliest extant group of seed plants with a minimum age in the Early Permian, they have been relevant for the study of phenotypic evolution between gymnosperms and angiosperms, origin of the seed, neurotoxins, as well as studies on coevolution (Norstog & Nicholls, 1997; Salzman et al., 2021). Today, 10 genera with a total of 368 species are recognized within Cycadales (Calonje et al., 2013–2023). During the last decades, the recognized diversity in some genera has increased dramatically, even though monographs and similar taxonomic treatments are scarce. In this article, we discuss the history of species discovery in cycads, species concepts, and trends in species delimitation over time. Finally, the causal processes that operate in the phenotype and genotype are addressed, which are co-responsible for the direction, rate and origin of the variation in the species.
Survey Methodology
A useful guide to basic terms is in Supporting Information S1. We used the literature database from “The World List of Cycads” (https://www.cycadlist.org) and Read and Solt (1986) as primary resources for the publications of all species and selected all taxonomic publications including floras and monographs to describe the trend of publication and trace the changes in the taxonomic concepts within Cycadales through September 2022.
To analyze the strategies and methods of empirical research focused on species delimitation in cycads, we used the following terms to select articles through September 2022 in the ISI Web of Knowledge: “new species”, “circumscription” “species delimitation” and “species complex” with “Cycadales” and each generic name in the order, i.e., Aulacophyllum Regel, Bowenia Hook. ex Hook.f., Ceratozamia Brongn., Cycas L., Chigua D.W.Stev, Dioon Lindl., Dyerocycas Nakai, Encephalartos Lehm., Epicycas deLaub, Lepidozamia Regel, Macrozamia Miq., Microcycas (Miq.)A.DC., Stangeria T.Moore and Zamia L. From this compilation, we selected the original studies of species delimitation published between January 1980 and August 2022 and conducted a systematic classification of the research for analytical criteria and methods of circumscription in Cycadales. We only selected the articles in which a method was explicitly included for sources of evidence as presented in each research presentation. In total, 41 articles have all the requirements for extensive analysis and those were reviewed in detail and classified according to the approach and criteria used by the authors (Supporting Information Table S2). The approach criteria and species concept were not explicitly indicated in most of these articles. For those cases, we classified according to the rationale used by the authors and issues of how they analyzed or integrated the results of sourced evidence obtained for the research. The articles were classified in three general approaches: i) traditional, ii) integrative taxonomy and iii) monophyletic. Historically, traditional taxonomy refers to studies that include only morphological data. However, molecular data analyzed independently is also part of this approach (Kotov & Gololobova, 2016). Basically, this approach has the descriptive perspective of defining features. We included all research in which the authors used at least two sources of evidence but without defined methods of analysis. Under the integrative taxonomy approach, we included articles with more than one source of evidence that was analyzed with the same or different methods for testing the species hypothesis. The monophyletic approach included studies based on tree methods because the species under this criterion are considered explicitly as historic lineages (Baum & Donoghue, 1995; Wiens & Penkrot, 2002).
To illustrate the importance of the morphological and molecular variation involved in taxa for species boundaries, we carried out a search for articles where the causes and processes involved at the level of the phenotype and genotype in cycads are addressed. In order to make recommendations and discuss future directions, we made a selection of these articles based on variety of methods and results that could be useful for new approaches in species delimitation.
Alpha Taxonomy: From Linnaeus to Phylogenomic
The formal taxonomic history of Cycadales began with the description of Cycas circinalis by Carl von Linnaeus (Linnaeus, 1763). The rate of species description in the order has not been constant. After a long period of 75 years, the publication of taxa currently accepted published and names published suddenly increased (Fig. 1). Overall, two peaks of descriptions were observed: (i) 1837 to 1880 and (ii) 1959 to 2004 (Fig. 1). The period of 1959 to 2004 is the historical maximum in which the number of described taxa were doubled compared to the period of 1900 to 1950. The current trend shows that taxa discovery has increased by nearly double over the last 50 years, which could indicate that almost all species of the order have been discovered and described (Fig. 1). Actually, several names published between 1875 and 1925 are currently considered synonyms or invalid names (Figs. 1 and 2; Supporting Information Fig. S3). The abrupt increase between 1925 and 1950 is derived from Schuster’s (1932) monograph where 86 new varieties, subspecies and species were described, but none these have names were not accepted in subsequent works (Fig. 1). We noted that more than half of the scientific names in cycads were published between 1850 and 2004; thus, 85% of scientific names were described before 2005 (Fig. 1; Supporting Information Fig. S3).
There are two periods (1837 to 1880 and 1959 to 2004) of increase in species descriptions that match the publications of monographic works at the genus level and broad geographic areas. The first period included the publication of six relevant monographic works prepared by Miquel (1842, 1861, 1863, 1869a, b, 1870), Regel (1875) and de Candolle (1868). The second period is characterized by the regional taxonomic treatments published for Cycas and Macrozamia (Johnson, 1959, 1961; Hill, 1994a, b, 1996; Wang, 1996; de Laubenfels & Adema, 1998; Hill & Yang, 1999). Most of the described species for these genera were published in these works. Half of the currently accepted taxa in Cycas and Macrozamia are synonymous or invalid and 50% and 44% of the names are accepted, respectively (Fig. 2c, g). In Cycas, several infraspecific categories such as varieties and subspecies have been described. However, most of the names described within this period were at the species level with very few at infraspecific levels (Supporting Information Fig. S3). Identification of species in Cycas has been complex by the great variation in the characters usually used for species identification. In particular, C. rumphii Miq. and C. circinalis L., have a long history of synonymy (Hill, 1994b, 1995a; Lindstrom, 2002; Hill et al., 2004). The publication pattern of names and taxa currently accepted in Cycas was regular, whereas in Macrozamia was irregular (Fig. 2; Supporting Information Fig. S3). Until 1940 Cycas showed a trend of constant increase in currently accepted taxa with an abrupt increase during the last thirty years (Fig. 2c). The trend in Macrozamia shows three intervals without new names published during 1900 to 1990 (Fig. 2g; Supporting Information Fig. S3). In this period of 90 years, Schuster (1932) introduced 26 scientific names. Most of these names were largely the cause of much of the confusion in this genus and overestimation of its diversity (Johnson, 1959). The number of currently accepted taxa published in this genus doubled in the last 40 years, and since 2000, only one taxon has been described.
The tendency in the first period (1837–1880) of cycad discovery was to recognize geographically localized species, particularly in Encephalartos and Zamia. This led to rapid increases in new taxa descriptions in these genera by incorporating the trends mentioned below (Figs. 1 and 2). Both genera show two periods of growth during 19th (1860–1880) and twentieth centuries (1980–1990) with more than half of the currently accepted taxa published before 1980 (Figs. 2e, j; Supporting Information Fig. S3). Several names were published as varieties and currently half of those are considered synonyms (Supporting Information Fig. S3). Zamia showed an upward trend in currently accepted taxa between 1763 and 1940 (Fig. 2j). The description of currently accepted taxa remained almost unchanged for 40 years with no new Zamia names published from 1960 to 1980, and 48 new names have been published in the last 40 years (Fig. 2j). However, the trend in number of names published was steady (Supporting Information Fig. S3). The publication of scientific names from 2000 has been characterized by the explorations of new areas in the mountainous region of the countries of South and Central America that had remained relatively inaccessible until the twentieth century (e.g.Calderón-Sáenz & Stevenson, 2003; Calonje et al., 2018; Segalla et al., 2023). In general, a minority of less that 10% of the species have been described based on a reassessment of morphological characters or populations previously considered part of other species (e.g. Calonje et al., 2010; Lindstrom et al., 2013; Nicolalde-Morejón et al., 2019). In relation to Encephalartos, most (96%) of the currently recognized diversity was described by about 2000. After this year, only 5 currently accepted names were published (Fig. 2e) and two that are currently synonyms were published (Supporting Information Fig. S3).
Dioon and Ceratozamia have a similar trend that shows a gradual increase of currently accepted taxa published from the description of the genera with an interval where no species were described (Fig. 2b, d). Two inactive periods of 50 and 40 years were registered for Ceratozamia with no new taxa described. The first period occurred between 1880 and 1930 and the second occurred from 1940 to 1980 in which only two species were described (Fig. 2b). The publication rate of taxa in these genera has accelerated in the last two decades, in which 50% of new taxa have been described. The descriptions of these species were based on re-circumscriptions of some taxa and/or reassessments of the range extension of widely distributed species. On the other hand, new taxa publications in Dioon increased in the decade of 1980 to 1990 where 30% of the total species were described (Fig. 2d). Unlike Ceratozamia, most names published in Dioon up to 1900 are now synonyms (Supporting Information Fig. S3). Similarly, as in Cycas, there was a steadily increasing trend in the number of taxa currently accepted published in Ceratozamia and Dioon with the greatest increase in the 1980–2020 period with a lull during a short period of 20 years during 1950 to 1970 (Fig. 1). The rest of the genera are monotypic or currently have only two currently taxa accepted. The publication of names in Stangeria, Lepidozamia and Microcycas has remained unchanged from the nineteenth century (Fig. 2f, h, i).
By 2000, 88% of species in Cycadales had been discovered and described (Fig. 1). The more recent descriptions were derived from reassessments of known taxa and/or populations that have been identified and segregated as new species (e.g. Gutiérrez-Ortega et al., 2020b; Martínez-Domínguez et al., 2022). By 1999, 78% of the cycad species had been described with several of these species described in regional taxonomic treatments and Floras (Vovides et al., 1983; Stevenson, 1993, 2001, 2004).
In Cycadales taxonomy, the works of Miquel and Regel have been among the most historically relevant. Miquel published 61 binomials under his authorship, of which 15 correspond to currently accepted species. In 1842, in the Miquel’s “Monographia Cycadearum”, four new species and 15 new varieties in four genera were described. He continued to publish new species and varieties in subsequent years, particularly in 1847 when he described eight species and one variety. After 1842, the recognition of varieties decreased considerably and by 1868, Miquel's species concepts were more inclusive. Miquel in his Prodromus systematis Cycadearum proposed a classification with four tribes and eight genera in 1861 and later also added an infrageneric classification for Macrozamia (Miquel, 1868). Regel published 39 names of which five correspond to binomials of currently accepted species. Most of the names published throughout his taxonomic works were varieties. However, Regel described two genera Aulacophyllum by transferring species from Zamia while describing a new species and Lepidozamia as a segregate from Macrozamia (Regel 1857a, b, 1876a, b). All species in the former are now considered a synonym of Zamia and the latter is currently accepted. In general, Regel’s early work had a focus on horticulture that included a list of species and notes on those species. Regel’s later works were more extensive and included taxonomic details and keys. In 1876, he published parallel taxonomic works in Horti Petro and Gartenflora in which he recognized varieties by other authors and proposed 15 new varieties of his own.
The works of Miquel and Regel show differences in some concepts and circumscriptions. In particular, Encephalartos in Miquel’s “Monographia Cycadearum” (1842) and Regel’s “Cycadearum generum specierumque revisio” (1876a), 11 scientific names were addressed in each of these monographs. These taxonomic works are characterized by the proposal of several infraspecific categories at the variety level. The varieties proposed between both treatments show conspicuous differences to each other. The same species addressed in these treatments have a different circumscription. For example, Miquel (1842) recognized E. altensteinii Lehm., with three varieties, whereas Regel (1876b) recognized five different varieties from which three were new proposals, and the “Miquelian names” were placed as synonyms. Under those outlines, the taxonomic concepts for the subspecific categories are incongruent with each other. Additionally, the number of recognized species differed in the final publications of Miquel (1868) and Regel (1876a, b) on cycads, 68 and 57 species, respectively.
The taxonomic concepts of the species have been subject to significant changes derived from the reevaluation of morphological characters and the relevance of these to designate categories (Miquel, 1842, 1848; Schuster, 1932). This has led to subsequent reassignments of the relationships among the historical taxonomic concepts of some binomials. In Macrozamia, the taxonomic concepts have been relatively stable and consistent with each other; however, due to its morphological similarity to Encephalartos, several transfers took place between these genera particularly by von Mueller (1858, 1859) where he lumped Macrozamia into Encephalartos, which was not accepted by others (Miquel 1861, 1868; Regel, 1876a, b; de Candolle, 1868; Schuster, 1932). Another genus with a taxonomic history with few changes and misconceptions is Dioon. This genus is one of the few that has been fairly clearly understood from its beginning with little misapplication of names.
Overall, the taxonomic concepts of species have been narrower such as Dioon tomasellii de Luca, Sabato & Vázq.Torres, D. sonorense (De Luca, Sabato & Vázq.Torres) Chemnick, T.J.Greg. & Salas-Mor. and D. merolae de Luca, Sabato & Vázq.Torres. Dioon merolae was proposed as a narrow concept; however, that taxonomic concept was extended during a revaluation of populations discovered during the late 1990s. Recently, two new species, D. oaxacensis Gut.Ortega, Pérez-Farr. & Vovides and D. salas-moralesiae Gut.Ortega & Pérez-Farr., were segregated from the broad concept of D. merolae (Gutiérrez-Ortega et al., 2020b, 2021). Similarly, Dioon tomasellii de Luca, Sabato & Vázq.Torres was proposed with two varieties from western Mexico. These varieties were described as D. tomasellii var. tomasellii and D. tomasellii var. sonorense, posteriorly these varieties were associated to the taxonomic concept of D. edule not without some ambiguity (McVaugh & Pérez de la Rosa, 1992). Both varieties of D. tomasellii, following these authors, were conceptually part of a broad concept proposed for D. edule that included D. tomasellii and their varieties. Thus, species concepts in Dioon were aggregated to form a more inclusive taxonomic concept. However, the taxonomic concepts were again reduced by the subsequent authors for which D. sonorense (De Luca, Sabato & Vázq.Torres) Chemnick, T.J.Greg. & Salas-Mor. and D. tomasellii are congruent with the originally described varieties. More recently, D. stevensonii Nic.-Mor & Vovides was segregated and described from populations previously considered to be D. tomasellii (Nicolalde-Morejón et al., 2009b).
Taxonomy in Cycadales has a complex history marked by the multiple synonyms that show a clear taxonomic disagreement at the species level (Supporting Information Fig. S3). For example, Epicycas and Dyerocycas were separated from Cycas and subsequently synonymized because the characters using to segregate these genera after a closer examination were found to be inconsistent (Chen et al., 2004). On the other hand, the taxonomic history within most genera is characterized by mutually independent species descriptions and the recognition of numerous infraspecific categories (sub-species, varieties, among others). This is clearly seen when looking at the number of published species names when compared to currently accepted taxa (Fig. 1). Encephalartos, Cycas and Zamia have the highest number of synonyms (Hill, 1995b, 1998, 2008; Hill & Yang, 1999; Nicolalde-Morejón et al., 2009a; Calonje et al., 2013–2023). Currently, 65 species and six infraspecific categories (subspecies) are recognized in Encephalartos and there are 85 synonyms of which 41 are varieties, two subspecies and six forms. Cycas is the largest genus with 120 species and six subspecies and 99 synonyms. Zamia is the second most speciose genus with 83 species and five varieties and 84 synonyms. One of the major issues in the early cycad treatments was the lack of a type specimen concept as well as the lack of specimen citations. Johnson (1959) established a turning point for Australian taxa followed by Stevenson and Sabato (1986a, b) for the typification of neotropical cycad names. Similarly, this was done for African taxa by Vorster (2004) and the genus Cycas by Hill (1995a, b) and Hill et al. (2004).
The designation of several varieties, subspecies and forms is mainly due to the morphological similarity between species and the lack of vegetative morphological characters that could be used as diagnostic. Some of the long history of synonymy within the order are in genera with little diversity, as is the case of Lepidozamia, Stangeria and Bowenia. In the latter, two specific epithets with three varieties are recognized as synonyms and even with changes in category recognition, all the taxonomic concepts since 1912 are consistent with each other (Table 1). This taxonomic history shows synonyms of the same entity under the concepts of variety and species. In this case, the changes have been raised into a more conservative approach discarding the variations in some characters (Table 1) within individuals and populations.
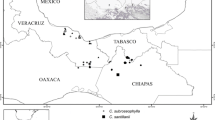
Ceratozamia has a complex taxonomic history in which C. mexicana Brongn., was one of the most difficult taxonomic concepts to clarify (Stevenson & Sabato, 1986b; Vovides et al., 2016; Martínez-Domínguez et al., 2018a, b, 2022). All species in this genus that were described up to 2004 were added within one of the seven species complexes proposed by Vovides et al. (2004). Recently, the taxonomic concept of C. robusta Miq. has been meaningfully changed from a broad concept employed by Miquel (1847) into a narrower concept by Martínez-Domínguez et al. (2022). Thus, this name is only partially congruent with its use in all previous taxonomic treatments (e.g., de Candolle, 1868; Miquel, 1868; Stevenson et al., 1986; Martínez-Domínguez et al., 2016; Gutiérrez-Ortega et al., 2021). This species had a wide range of distribution with several populations that have been reduced in the last 10 years. Considering the recent description of new species previously considered as part of C. robusta, this taxonomic concept could be confusing, but these changes have been described in a recent monograph (Martínez-Domínguez et al., 2022). Other species previously considered as varieties or forms were transferred to the rank of species. In most cases, the taxonomic concepts within Ceratozamia have been narrowed. In contrast, Zamia showed a tendency to a broad taxonomic concept. Zamia loddigesii Miq., is a species with a wide distribution range through which it presents morphological and molecular differences. From its description by Miquel (1843) to the twentieth century, 12 new names related to Z. loddigesii have been published. Regel (1857a, b, 1876a, b) published 4 names under the variety rank using the names of species previously published by Miquel (1843, 1847). Later, in Flora Centrali-Americana (Thiselton-Dyer, 1884) two new species were proposed and the synonyms under Z. loddigesii were rearranged and Schuster (1932) described one new species and designated several new varieties. Evaluation of morphological variation and geographic discontinuities in populations similar to Z. loddigesii led to the clarification in its taxonomic concept including all variations (Nicolalde-Morejón et al., 2009a).
Currently, infraspecific taxa are not used in most genera. Only Cycas, Zamia and Encephalartos have a classification that includes infraspecific ranks such as subspecies and varieties. After an assessment of intra- and inter-population variation, most of those varieties and subspecies described were not supported. The classification based on these ranks was discounted for lack of clarity as in Macrozamia where Schuster (1932) and Johnson (1959) used infraspecific ranks. Schuster (1932) proposed ranks of varieties and forms in his taxonomic that caused considerable confusion. In contrast, Johnson (1959) used subspecies that are now unequivocally recognized at the species level (Hill, 1998). Johnson (1959) provided thorough descriptions of morphological characters for identified variations and diagnoses and these concepts were adopted by Hill (1998) in his treatment of the genus in the Flora of Australia.
The definitions of variety and subspecies are still controversial and criticized for their apparently arbitrary nature. The use of subspecies is based on the slight differences that are present in at least one character among populations (Zachos, 2016c); however, subspecies and variety show no discernible clear differences. Beginning in the latter part of the twentieth century, variety is usually used for one population and subspecies includes more than one population (Hamilton & Reichard, 1992). These arbitrary classifications could be useful for identifying and studying discontinuities in those described biological entities. One problem with the use of these ranks is that splitting is based on different criteria being used with the most common being allopatric distributions. The uncritical acceptance of these infraspecific categories is an unnecessary burden when based purely on phytogeography. In cycads, variety was commonly used early and later replaced by subspecies. This was prompted because of limited sampling of a few plants in living botanical collections and gardens without herbarium vouchers. It is possible that some of these plants were actually not a healthy representation of wild populations. Some recent species discoveries have even been made from cultivated plants that had been introduced into horticulture in the USA such as Zamia splendens Schutzman. The critical issue in using these discontinuities in some cycad genera has led to species recognitions by changing the rank and/or without using more detailed information on morphological variation and other sources of evidence. The excessive use of this approach historically has resulted in problems in understanding the group and contributed to taxonomic inflation of infraspecific nomenclature. The scattered distribution of many cycad species could lead to taxonomic inflation if differences in populations are raised to species level without an integrated approach to data analyses.
Monographing the Cycad Diversity
Most taxonomic treatments at the ordinal or familial level were published during the nineteenth century (Regel, 1857a, b, 1876a, b; Miquel, 1861, 1868, 1869a, b; de Candolle, 1868; Thiselton-Dyer, 1884). All these works described and tested species hypotheses of the group, they also showed the difficulties related to vegetative similarities among and between species. The most inclusive monograph was published by Schuster (1932) in the Pflanzenreich series. This monograph overestimated species diversity because it described any morphological character as a different taxonomic entity. Some sort of difficulty in interpreting Schuster’s taxonomic treatment stems from his reliance of material housed in the Berlin Herbarium which cannot be consulted due to the destruction during the bombing of Berlin in 1943 during World War II. The use of photographs in species publications and digitization of type specimens at herbaria as is now being done would overcome these drawbacks. One major problem with his approach was the recognition of a large number of varieties and forms under each species. This led to establish vague species circumscriptions, lack of nomenclatural priorities and typification as thoroughly reviewed by Laurie Johnson (1959). That and the fact that Schuster did not use or designate types made the work even more enigmatic. Progress was made with the typification of New World taxa by Stevenson and Sabato (1986a, b) and Australian taxa by Johnson (1959). Since then, regional taxonomic treatments and individual species description papers have predominated in cycad taxonomic literature. In particular, Flora of Australia (Johnson, 1959; Hill, 1998) is a contribution that included all species described for Macrozamia, Lepidozamia and Cycas species in Australia. In the early treatment by Johnson (1959), nine new species were described in Macrozamia based upon careful evaluation of all available data and numerous herbarium and living collections. This work in particular has served to establish the evaluation of detailed comparative morphological data as well as distributional and ecological data.
Some regional Flora projects such as Flora del Bajío (Vovides, 1999), Flora del Valle de Tehuacán-Cuicatlán (Medina & Dávila, 1997), Flora Novo-Galiciana (McVaugh, 1992), Flora de Veracruz (Vovides et al., 1983) for Mexico, for the Guianas and Venezuelan Guayana (Stevenson, 1991b,c, 2006) and Flora of North America (Landry, 1993; Stevenson, 1991a) have contributed with descriptions, illustrations and distributional ranges of cycad species that occur in these areas. Other relevant flora treatments and that are more extensive in number such as Flora of Australia (Hill, 1998), Flora of China (Chen & Stevenson, 1999), Flora de Colombia (Stevenson, 2001), Panama (Stevenson, 1993) and the countries Bolivia, Ecuador, and Peru (Stevenson, 2004) contributed to the recognition of new species and tested several species hypotheses in Cycas and Zamia.
The regional taxonomic revisions (i.e., taxonomic treatments) contributed significantly to clarifying the identity of many described species from 1753, the starting date for nomenclature, and transferred some varieties, subspecies and forms to ranks of species (Fig. 3). Cycas is the genus with the highest number of taxonomic revisions because of its high diversity. It was mostly during the twentieth century that the diversity of Cycas was discovered and described (de Laubenfels & Adema, 1998). However, the taxonomic revisions from Vietnam, Philippines and China led to a 40% increase in known species within this genus during the 21th century (Lindstrom & Hill, 2007; Hill, 2008; Lindstrom et al., 2008). Although revisionary work is still needed in this diverse genus, all these works plus Flora of China provide a solid base for a future monograph of the genus.
Zamia and Encephalartos have been scarcely addressed through comprehensive taxonomic treatments. Zamia has had a slow but steady increase of new species since 1842 with several species published from different countries by various authors. The most inclusive treatments focused on broad geographic regions such as Colombia, Panama and Mega-Mexico as well as Bolivia, Ecuador and Peru were particularly relevant in clarifying the historical species concepts, providing dichotomous keys as an effective means to identify species, and data to recognize hitherto undescribed taxa as well as more extensive specimen citations (Stevenson, 1993, 2001, 2004; Nicolalde-Morejón et al., 2009a). This encouraged the discovery and description of some new species from Mexico and South America during the last decade, which significantly increased the diversity of Zamia (e.g. Calonje et al., 2011; Nicolalde-Morejón et al., 2019). In contrast, a modern complete taxonomic revision is lacking for Encephalartos, which is essential to test the species hypotheses raised in the genus over the last century (Fig. 3).
Dioon has remained without significant taxonomic rearrangements in the twentieth century. Most species were discovered and described through botanical explorations in Mexico. These individual descriptions provided an understanding of morphological variation that raised good foundations for the taxonomy within the group. Recently, a taxonomic revision of Dioon was published using the examination of herbarium specimens (Hernández-Tapia et al., 2020), which has provided a reference of integrated taxonomic information from several previous taxonomic works within this genus by different authors. This treatment was revisited with a focus on some inconsistencies related to the diagnostic characters and nomenclature (Haynes, 2020). However, in general, these works did not change the taxonomic concepts of Dioon species.
In Ceratozamia, the early names published by Miquel (1847) remained synonyms for decades including in the later works of Miquel (1868–1869). Several varieties and forms were proposed in the infraspecific level taxonomy in the monograph of Schuster (1932) and Flora de Veracruz by Vovides et al. (1983). The recent regional taxonomic treatments from Sierra Madre Oriental (Mexico) and its circumscriptions aided in the discovery of new species and clarified the use of names that were typified in 1986b by Stevenson and Sabato (Martínez-Domínguez et al., 2016, 2017, 2018a; Vovides et al., 2016). More recently, the monograph published for this genus is the first comprehensive treatment for this genus in the last century and doubled the number of species during the last forty years (Martínez-Domínguez et al., 2022; Fig. 3).
Regional taxonomic treatments have treated more than 80% of the species described to date in most of the world’s genera (Fig. 3). Together these efforts have demonstrably aided progress monographing the diversity of cycads. The combination of these projects with taxonomic reviews and monographs at the genus level will be the cornerstone for future research on cycads. Some Flora projects were published when the diversity of regions and genera were poorly explored and that could now be updated and revised such as Flora de Veracruz (Vovides et al., 1983). In this floristic treatment, only one Ceratozamia species with three varieties were recognized based upon available data, specimens and knowledge at that time. Now, based upon using this treatment and subsequent data and collections, there are currently six different species described and recognized for Veracruz (Martínez-Domínguez et al., 2022; Fig. 3). In contrast, considering that Macrozamia is a genus with few taxonomic modifications and only one new species described since the 2000, the Flora Australia remains up to date (Hill, 1998). Monographs and flora treatments provide the framework for facilitating future knowledge and new species discovery when they cannot be identified in those works. These projects lead to evaluation and eventually updated treatments when the data and collections have been increased significantly.
Species Delimitation: Discovery Strategies and Description of Diversity
Species delimitation studies were only found in Ceratozamia, Cycas, Dioon, Macrozamia and Zamia. The most used approach was the traditional, which was used in these five genera (Table 2). From all approaches six species delimitation criteria were detected (Table 2). Even so, a species concept was not explicitly stated in the taxonomic works. The criteria and methods used imply that the researchers have used an ontological definition of species (Sites & Marshall, 2004). From non-tree-based methods, diagnosability, phenetic, genetic and niche specialization criteria were detected. Historically, the diagnosability criterion has been related to the use of morphological characters to recognize species. This criterion is based on the patterns of discontinuities in observable characters to establish limits between species (Sites & Marshall, 2004). From these considerations, both diagnosability and phenetic criteria share a common history. However, the diagnosability criterion recognizes the presence of both morphological and molecular character states to delimit a species (Cracraft, 1983). This criterion has predominated in the discovery and description of cycad species. The morphological evidence has been applied only descriptively, whereas the molecular data have been analyzed through DNA barcoding (e.g. Little & Stevenson, 2007; Nicolalde-Morejón et al., 2009b; Calonje et al., 2018; Martínez-Domínguez et al., 2020) and molecular phylogenetic approaches at the genus level (Liu et al., 2018; Habib et al., 2022).
The phenetic criterion has been used in all the genera of Cycadales (Table 2). This criterion is based on quantifying shared similarities between individuals by assigning a numeric value that allows to recognize similarities or dissimilarities among species (Sokal & Crovello, 1970). Several statistical methods are used in this criterion with the clustering of individuals and populations according to similarities through distance analyses. Some examples are sorting methods such as Principal Component Analysis (PCA) and canonical correlation analysis (Sites & Marshal, 2004). This criterion is applied to both morphological and molecular data and but is most commonly used with vegetative morphological characters in cycads (Table 2; Supporting Information Table S2). Only in Zamia has this criterion been used with qualitative evidence discretized using PCA (Nicolalde-Morejón et al., 2008). Also, UPGMA is a widely used phenetic method in which through Euclidean distances the differences between groups are calculated and visualized in similarity phenograms (Saitou & Nei, 1987). Generally, molecular data have been analyzed using this method from one individual per population of each species under circumscription in Ceratozamia (Pérez-Farrera et al., 2017) up to multiple individuals per population of each species as in Macrozamia (Sharma et al., 1998).
The genotypic cluster criterion is based on the degree of mixture among populations. Thus, this method evaluates the genetic subdivision and analyzes the absence of genetic intermediates (Mallet, 1995; Sites & Marshal, 2004). The PCO-MC method was one of the first proposed approaches (Mallet, 1995). Recently, other methods have been implemented using the genetic information to analyze potential contact between putative populations or groups such as in Hardy–Weinberg equilibrium (Huelsenbeck et al., 2011; Carstens et al., 2013). Here, we considered the methods based on these genetic intermediates such as those that describe the genetic structure of populations (e.g. Radha & Singh, 2011). Overall, this method in Cycadales has been used as a descriptor of genetic variation and to propose putative genetic groups that are later tested by other methods like Bayesian or coalescent (Table 2).
All the previous methods have in common an orientation towards the variation of characters, whereas the niche specialization criterion is the interaction between the environmental variables in the species habitat. This criterion consists of modeling the ecological niche of the species based on the geography and habitat description of the species (Wiens, 2007). Thus, the method analyzes whether the ecological niches of the species overlap or diverge (Schoener, 1968; Warren et al., 2008). This criterion has been applied in Dioon and Cycas as complementary evidence (Mudannayake et al., 2019; Gutiérrez-Ortega et al., 2021) and the ecological data were used with other evidence sources for testing species hypotheses (Table 2; Supporting Information Table S2).
The phylogenetic and coalescent criteria are based on tree methods and are oriented to the study of patterns between species (Table 2). In the first criterion, there are phylogenies based on maximum parsimony, maximum likelihood and Bayesian inference (Goloboff, 2003). The method more commonly used in cycads is the Bayesian method (Supporting Information Table S2). Also, methods as proposed by Brower (1999) are included in this criterion, which implies reconstructing a phylogeny of haplotypes looking for parsimonious patterns to test the hypotheses of species recognized a priori. Their principles of phylogenetic reconstruction are relations between species, whereas in the coalescent criterion is tokogenetic relations (Knowles & Carstens, 2007; Degnan & Rosenberg, 2009). The latter criterion has been hardly applied in Cycadales, within Cycas and Dioon (Table 2).
In our review of literature, only 17% of species delimitation studies in Cycadales presented a taxonomic treatment (Table 2). Of this total, 34% presented typification of the names, but did not provide a botanical description for any of the species addressed. Most studies made taxonomic recommendations as synonyms or new species, but not a formal proposal (Supporting Information Table S2). These results are congruent with the prevailing trend in species delimitation studies (Carstens et al., 2013). Our content analysis suggests two potential explanations for this situation: (i) limited support for the species hypotheses and (ii) the lack of morphological evidence for describing the species. This last point is related with the lack of an evaluation of reproductive morphological evidence at population level (Supporting Information Table S2). In general, the morphological data and the review of herbarium specimens are basic in carrying out taxonomic revisions. The taxonomic proposals as descriptions of new species or synonymy were made in fewer than 30% of studies in Cycadales (Table 2).
Also, we found species delimitation studies that only discussed the possibility of more or fewer species in a species complex but without confidence in the data and methods used because the taxa were not clearly recircumscribed species (e.g. Medina-Villarreal & González-Astorga, 2016). Two species delimitation studies were found in which new species were suggested, but the taxa were described in another journal (Gutiérrez-Ortega et al., 2018a, b, 2020a, b). This is consistent with observations of several authors who have suggested a crisis in the value that the scientific community gives on traditional descriptions of new species (Wheeler et al., 2004; Carstens et al., 2013; Grace et al., 2021; Wheeler, 2023). The taxonomic treatments and species descriptions based on circumscription are time consuming and most are published in journals with a low impact factor (Carstens et al., 2013). Unfortunately, this is due, in part, to the fact that the papers are cited in the actual nomenclatural treatment but not in the literature cited. Thus, they have low citation statistics. This could lead, in turn, to practices that waste valuable research time, sources and new methods of interest for circumscription, and without clarifying many of the species included in species complexes. Conversely, it is important that evolutionary biology research papers who discover the impact of their results on species circumscription publish formal taxonomic proposals according to the International Code of Nomenclature for Algae, Fungi, and Plants (Turland et al., 2018). Moreover, synonymizations should be formally proposed by including them and their types under a proposed accepted name.
Species Concepts: Meaning and Change
The literature of species concepts is vast and several species concepts have been proposed (Zachos, 2016a). Each species concept is a definition of the species category with implied different levels of inclusiveness (Zachos, 2016c). According to the species concept adopted, the biological entities that the authors are studying should be more or less inclusive. In Cycadales, there is a plurality of species concepts some of which are not compatible in terms of their definitions and scopes. This situation has led to the described biological entities being inconsistent with each other, for example, the species included within the Ceratozamia miqueliana complex (c.f. Martínez-Domínguez et al., 2017; Vovides et al., 2020) and Zamia splendens vs Z. katzeriana (c.f. Nicolalde-Morejón et al., 2009a; Pérez-Farrera et al., 2016). These concerns were pointed out by cycad botanists as the need to incorporate the new tools available within the classification at the species level and avoid taxonomic inflation (Norstog & Nicholls, 1999). Historically, the term “morphogeographic” has been proposed as a species concept in cycads (see Schutzman, 2004; Whitelock, 2004; Pérez-Farrera et al., 2021). It is defined as a population or group of geographically isolated individuals with morphological characters that allow them to be differentiated from other individuals (Schutzman, 2004). Overall, it assumes a geographic component but excludes sympatric speciation as a process. According to theoretical reviews and debates on the concepts of species and their definitions, it was found that the definition and interpretation of this term in taxonomic studies in cycads corresponds with the biological or phenetic concept of species (De Queiroz, 2007; Zachos, 2016a, b).
Most descriptions of new species in Ceratozamia suggest geographic isolation, a property of the biological species concept (e.g. Pérez-Farrera et al., 1999, 2009; Vovides et al., 2004). This concept is based on species as individuals that are interbreeding and reproductively isolated from other groups (Sokal & Crovello, 1970; De Queiroz, 2007). Recently, Pérez-Farrera et al. (2021, p. 243, 252) highlighted the use of the morphogeographic concept. Nonetheless, these authors focused on methods from numerical taxonomy for discovery that coincide with the phenetic species concept (Pérez-Farrera et al., 2021). The phenetic concept is defined as groups of individuals with quantifiable differences that allow them to be recognized as a biological entity (Sneath, 1976). Also, apparently, this concept was applied by Medina-Villarreal & González-Astorga (2016); however, the authors point out “taxonomic species concept” (Medina-Villarreal & González-Astorga (2016, p. 213), which was defined as a population(s) similar in morphological characters within a delimited geographic area that differ(s) from other species. Based on these properties and the methodological procedure through numerical methods using quantitative and qualitative morphological characters analyzed for 13 populations of 4 species by uni- and multivariate techniques, we could infer the phenetic species concept in this study (Medina-Villarreal & González-Astorga, 2016).
Recently, the phylogenetic concept of species has been used in Ceratozamia. This species concept has been defined from different perspectives; however, all show greater or lesser degrees of concordance in considerer a species as an aggregation of populations or lineages diagnosable by an exclusive combination of character states (Cracraft, 1983; Baum & Shaw, 1995; Meier & Willmann, 2000; Wheeler & Platnick, 2000). Some traditional and integrative taxonomy approaches have a theoretical framework and inferential procedure of the phylogenetic species concept (e.g. Stevenson et al., 1986; Martínez-Domínguez et al., 2016). On the other hand, the evolutionary concept considers a species as a lineage with its own evolutionary tendencies and historical fate (Wiley & Mayden, 2000; de Queiroz, 2007). A recent phylogenetic study based in six loci with one individual per species analyzed by Bayesian Inference proposed a circumscription for the genus of 30 species, of which there would be two new species, seems to be framed in the evolutionary concept (see Medina-Villarreal et al., 2019). However, the authors stated a history-based approach where species are defined from coalescent theory and recognized as independent evolutionary lineages (Vovides et al., 2020, p. 12).
The biological species concept has been the most commonly one applied in Cycadales. This species concept has been used in most species of Zamia, with few exceptions where the phenetic species concept was applied (e.g. Pérez-Farrera et al., 2016). Generally, the phylogenetic species concept and the biological species concept have been applied in Bowenia, Encephalartos, Lepidozamia, Macrozamia, Microcycas, and Stangeria. In Cycas and Dioon, the ecological species concept has been explored in which a species is defined as biological entity that possesses and evolves to the same niche or adaptive zone (Van Valen, 1976; e.g. Gutiérrez-Ortega et al., 2020a). More recently, the phylogenetic species concept under genealogical criteria has been raised in these genera (Table 2).
In the species problem, the unified species concept and the hierarchical species concept have been postulated as approaches that reconcile the dispute between species concepts (de Queiroz, 1998, 2007). These species concepts do not introduce a conceptualization of species in nature. Conversely, they are models based on the main principles of the evolutionary species concept and converge in their vision of species as evolutionary lineages (Mayden, 1999; Naomi, 2011). In practice, these concepts are commonly used for studies of delimitation or description of new species under integrative approach, but in the circumscriptions under this approach in cycads, the species concept has not been clarified or discussed. The unified species concept and hierarchy species concept could be alternatives to the species problem because they represent integrated frameworks that conceptualize species in the evolutionary context (Zachos, 2016b). It is imperative on researchers to clearly state species concept of their investigations. Thus, statements can also establish the appropriate level of inclusiveness in delimitating species. Under these considerations, integrative taxonomy would provide a robust epistemological framework for delimitation.
In some genera in Cycadales, the use of species concepts has been ad hoc. There is no consensus on which species concept clearly describes the biological entities, but the consistency and use of species concepts according to their conceptualizations will allow a robust framework in the species hypothesis (Rieppel, 2009; Zachos, 2016a, b). Although the species delimitation implies a degree of arbitrariness, a solid understanding of the species concepts and the methods would allow to conduct a species delimitation investigation consistent across the results. This is necessary to avoid artificial conservation proposals or evolutionary inferences (Zachos, 2016c).
Phenotypic and Genotypic Variation: Implications for Species Delimitation
Species are spatio-temporally located lineages that evolve at different scales and times through different evolutionary processes (Rieppel, 2009; Goldstein & DeSalle, 2011; Zachos, 2016b). Therefore, species delimitation is a complex systematic task. The detection of patterns at the level of the phenotype or genotype of individuals is crucial in species boundaries. In turn, these patterns are essential to study and understand the evolutionary processes underlying to the current diversity of species (Wake et al., 2011).
The phenotypic patterns detected in Cycadales have been convergent in vegetative morphological characters, morphological polymorphisms and morphological stasis including the retention of ancestral characters (Brenner et al., 2003; Calonje et al., 2019). At the genotypic level, different studies have highlighted low levels of genetic divergence between species (Brenner et al., 2003). The patterns and inference of causal processes that interact at the phenotypic and genotypic levels have been approached from different angles of research (Supporting Information Table S4). Evolutionary processes directly change phenotype and genotype in generating variation or modifying the frequencies of heritable variation (Laland et al., 2015). Evolutionary processes such as genetic drift, gene flow and natural selection are powerful agents on organisms at population level, which leads to modifying potentially heritable variation (Supporting Information Table S4). Additionally, heritable epigenetic variation induced by environmental changes has potential influence on adaptation and, thus, speciation.
The current diversity of cycads is the result of a recent adaptive radiation process during Miocene-Pleistocene in which vicariance played a relevant role (Nagalingum et al., 2011; Liu et al., 2018). The long-range dispersal events are rare, but have occurred and the climatic conditions appear to have limited its expansion due to conservation of ecologically suitable conditions (Liu et al., 2018; Gutiérrez-Ortega et al., 2021). The processes directly related to the origin of variation at the genetic level are recombination and mutation (Supporting Information Table S4). The correlation of chromosomal fission with the environment and morphology has been studied in Zamia (Jones, 1998; Moretti & Sabato, 1984; Olson & Gorelick, 2011). Most of the genera have conservative morphological evolution (Brenner et al., 2003); however, there is high variation of vegetative characters within Zamia, which has been attributed to this process. Recently, genome duplication was proposed as a process in gymnosperms that is linked to the generation of innovation in the phenotype (Stull et al., 2021).
Because cycads are characterized by slow growth and a long life cycle, they are not a model group for this type of evo-devo studies. Nevertheless, recent approaches have explored the processes at this level and their regulatory networks during cycad development, such as expression of MADS-box and KNOX1 genes involved in the control of reproductive growth and in the control/regulation of cell identity in the shoot apical meristem, respectively (Supporting Information Table S4). On the other hand, epigenetic factors could play a relevant role on the phenotype and genotype (Laland et al., 2015). Accordingly, the influence of DNA methylation on the cycad phenotype have been studied from pioneering approaches in Cycas (Sae-Eung et al., 2012).
Studies on environmental changes in Cycadales found that the aridification process, and the climatic and environmental changes temporarily (on a geological scale) could promote phenotypic variation in both the macromorphological and micromorphological levels (Supporting Information Table S4). In particular, the interspecific variation detected in anatomical characters in Dioon has allowed us to infer how environmental changes such as volcanism and water stress might have driven phenotypic expression (Barone-Lumaga et al., 2015; Gutiérrez-Ortega et al., 2018b). Also, the high intraspecific variation among populations of Zamia species were related to climatic conditions in the area where those species occur (Limón et al., 2016).
A relevant issue in cycads is reproductive phenotype, which has attracted considerable attention due to several characters such as the sarcotesta of seeds and simple strobili (Brenner et al., 2003). All genera have sporophylls arranged in a determinate central axis, i.e., a simple strobilus. The exception is the ovulate plants of Cycas where there is a spirally arranged overlapping indeterminate set of megasporophylls (Brenner et al., 2003). Although the reproductive structures occupy a key position in the phenotype, few studies have examined the intra and interspecific variation within genera as well as the role of the causal processes that have influenced the current variety of sporophyll shape. Even though phenotypic plasticity has been omitted from studies on vegetative morphology, it could a key on the divergence and origin of the phenotype (c.f. Medina-Villarreal et al., 2019; Vovides et al., 2020). Recent studies proposed niche conservatism as a process that can promote speciation in cycads (Gutiérrez-Ortega et al., 2021). However, this tendency of closely related species to occupy similar niches represents a pattern (Crisp & Cook, 2012). New approaches that enable investigation of this pattern in Dioon could be focused on exploring the causality of the underlying processes between climate pattern and morphology. For instance, geographical and/or ecological allopatry is a driver of speciation at the genotypic and/or phenotypic level (Supporting Information Table S4). The response of populations to new habitats implies a multidimensional model.
In addition to the above, mutualistic pollination systems could contribute to species diversification. Pollination is mediated by highly specialized pollinating insects where chemical communication is a mechanism in this coevolution (Salzman et al., 2021). New inferences on morphological convergence of diaspores in gymnosperms as a result of the dispersal process show how multiple processes interact at different times during the evolution of species (Contreras et al., 2017).
The developmental processes such as neoteny, progenesis, peramorphosis and paedomorphosis were proposed in Cycadales (Carpenter, 1991; Coiro et al., 2021). The first two processes were studied using the morphological comparison among species of Macrozamia and Bowenia (Supporting Information Table S4) with respect to peramorphosis and paedomorphosis as addressed in Ceratozamia (Medina-Villarreal et al., 2019) where the research focused on the approach of ultimate and proximate causes according to Mayr’s proposal in 1961 (Supporting Information Table S4). This approach is on the rise in current debates because separation among proximate causes with ontogeny and ultimate causes with phylogeny that arise from evo-devo, developmental plasticity, inclusive inheritance and niche construction as part of the extended evolutionary synthesis (Laland et al., 2011). The duality of phenotype and genotype converges in the ontogenetic processes of individuals and have an impact on the divergence of species. In particular, the environment has a direct and indirect effect on the observable characters above the molecular level and the genes of the species. This includes both the physical environment (soil, temperature, etc.) and biotic interactions (Laland et al., 2011, 2015).
An alternative to avoid one-way bias is reciprocal causality where there is no dichotomy between ultimate and proximate causes (Laland et al., 2011). This approach requires an understanding of preexisting developmental processes and assumes that these are interacting with genetic, environmental, and epigenetic factors. Additionally, the characters that are acquired, including phenotypic plasticity, are not the direct result of the proximate causes of an individual, but rather the origin of selection and variation (Laland et al., 2011, 2015). Our understanding of mechanisms underlying the evolution in cycads has increased with genomics and new approaches in the study of the phenotype. Integrate and synthesize the actual phenotypic and genotypic knowledge of cycads could lead to benefits and provide information relevant for the species delimitation using modern approaches with a solid base in taxonomy.
Conclusions and Perspectives
Accurate species delimitation integrating multiple methods and data sources will establish a solid taxonomy. Under this concept, modern monographs in Cycadales should and could be developed and set a standard for the disseminate of scientific information from taxonomic data, for example, such as descriptions, distributions, ecological data and even photographs. Such monographs will impact other fields such ecology, genetics, conservation and natural resource management in this group, which is considered to be at a high risk of extinction.
We emphasize the importance of species delimitation with taxonomic treatments based on an examination of a consistent number of specimens and exhaustive evaluation of characters variation within and between species. We particularly discourage species splitting based on only in quantitative characters and one population without both morphological and geographical circumscriptions because the species are highly variable at intrapopulation level. Ideally, we recommend the approach that integrates information from different sources including rigorously handling data from prior taxonomic research and nomenclature. The explanation of cumulative information on a scientific name is necessary for species subject to significant changes in past so that the taxonomic legacy associated with that name is not lost.
We further stress the value of the identification of voucher specimens that are deposited in public institutions provided by the authors of the monographs. As such, these results make the information accessible to other biological disciplines. Finally, our review suggests several approaches for further research to address causal processes in the evolution within Cycadales. In particular, more focus is needed on reproductive structures in the evolutionary trends of cycads. We advocate those approaches that enable studies across the different scales from genes to ecology.
Data Availability
All data is available either in the article or as online supplementary information.
References
Bailey, F. M. 1883. A Synopsis of the Queensland Flora. 501.
Barone-Lumaga, M. R., M. Coiro, E. Truernit, E., B. Erdei & P. de Luca. 2015. Epidermal micromorphology in Dioon: did volcanism constrain Dioon evolution? Botanical Journal of the Linnean Society, 179, 236–254. https://doi.org/10.1111/boj.12326
Baum, D. A. & M. J. Donoghue. 1995. Choosing among alternative “phylogenetic” species concepts. Systematic Botany, 20, 560–73. https://doi.org/10.2307/2419810
Baum, D. A., & K. L. Shaw. 1995. Genealogical perspectives on the species problem. In P. C. Hoch & A. G. Stephenson (Eds.), Experimental and Molecular Approaches to Plant Biosystematics (pp. 289–303). Monographs in Systematic Botany from the Missouri Botanical Garden, St Louis, Missouri.
Brenner, E. D., D. W. Stevenson & R. W. Twigg. 2003. Cycads: evolutionary innovations and the role of plant-derived neurotoxins. Trends in Plant Science, 8(9), 446–452. https://doi.org/10.1016/S1360-1385(03)00190-0
Brower, A. V. Z. 1999. Delimitation of phylogenetic species with DNA sequences: A critique of Davis and Nixon’s population aggregation analysis. Systematic Biology, 48(1), 199–213.
Calderón-Sáenz, E. & D. W. Stevenson. 2003. Una nueva especie del género Zamia L. (Zamiaceae), de los Andes de Colombia y clave actualizada para las especies del género en Colombia. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 27(105), 485–490.
Calonje, M., D. W. Stevenson, C. Calonje, Y. A. Ramos & A. Lindstrom. 2010. A new species of Zamia from Chocó, Colombia (Cycadales, Zamiaceae). Brittonia, 62(1), 80–85.
Calonje, M., H. E. Esquivel, D. W. Stevenson, C. Calonje & D. Pava. 2011. A new arborescent species of Zamia from the Central Cordillera of Tolima, Colombia (Cycadales, Zamiaceae), with comments on the Z. poeppigiana species complex. Brittonia, 4(1), 442–451. https://doi.org/10.1007/s12228-011-919
Calonje, M., C. Lopez-Gallego & J. Castro. 2018. Zamia paucifoliolata, a new species of Zamia (Zamiaceae, Cycadales) from Valle del Cauca, Colombia. Phytotaxa, 385(2), 85–93. https://doi.org/10.11646/phytotaxa.385.2.4
Calonje, M., A. W. Meerow, M. P. Griffith, D. Salas-Leiva, A. P. Vovides, M. Coiro & J. A. Francisco-Ortega. 2019. Time-calibrated species tree phylogeny of the New World cycad genus Zamia L. (Zamiaceae, Cycadales). International Journal of Plant Sciences, 180, 286–314. https://doi.org/10.1086/702642
Calonje, M., D. W. Stevenson & L. Stanberg. 2013–2023. The world list of cycads. http://www.cycadlist.org [Access 07.02.2023]
Carpenter, R. J. 1991. Macrozamia from the Early Tertiary of Tasmania and a study of the cuticles of extant species. Australian Systematic Botany, 4(2), 433–444. https://doi.org/10.1071/SB9910433
Carstens, B. C., T. A. Pelletier, N. M. Reid & J. D. Satler. 2013. How to fail at species delimitation. Molecular Ecology, 22, 4369–4383. https://doi.org/10.1111/mec.12413
Chamberlain, C. J. 1912. Two species of Bowenia. Botanical Gazette, 54, 419–423.
Chen, J. & D. W. Stevenson. 1999. Cycadaceae Persoon. In Z. Y. Wu & P. H. Raven (Eds.), Flora of China. Vol. 4 (Cycadaceae through Fagaceae) (pp. 1–7). Science Press, Beijing, and Missouri Botanical Garden Press, St. Louis.
Chen, C. J., K. D. Hill & D. W. Stevenson. 2004. Comments on Cycas, Dyerocycas, and Epicycas (Cycadaceae). In T. Walters & R. Osborne (Eds.), Cycad Classification: Concepts and Recommendations (pp. 57–68). CABI Publishing, Oxford.
Coiro, M., J. Nicola, H. Neuenschwander, M. A. Calonje, A. P. Vovides, J. E. Mickle & M. A. Barone-Lumaga. 2021. Evolutionary signal of leaflet anatomy in the Zamiaceae. International Journal of Plant Sciences, 181(7), 697–715. https://doi.org/10.1086/709372
Contreras, D. L., I. A. P. Duijnstee, S. Ranks, C. R. Marshall & C. V. Looy. 2017. Evolution of dispersal strategies in conifers: functional divergence and convergence in the morphology of diaspores. Perspectives in Plant Ecology, Evolution and Systematics, 24, 93–117. https://doi.org/10.1016/j.ppees.2016.11.002
Cracraft, J. 1983. Species concepts and species analysis. Current Ornithology, 1, 159–187.
Crisp, M. D. & L. G. Cook. 2012. Phylogenetic niche conservatism: what are the underlying evolutionary and ecological causes? New Phytologist, 196, 681–694. https://doi.org/10.1111/j.1469-8137.2012.04298.x
Cronquist, A. 1978. Once again, what is a species? In L. Knutson, (Ed.), Biosystematics in Agriculture. (pp. 3–20). Allehed Osum, Montclair, NJ.rrowson
Crowson, R. A. 1970. Classification and biology. Heinemann Educational Books, London.
de Candolle, A. P. 1868. Cycadaceae, in Prodromus systematis naturalis regni vegetabilis 16(2), 522–548.
de Queiroz, K. 1998. The general lineage concept of species, species criteria, and the process of speciation In D. J. Howard & S. H. Berlocher (Eds.), Endless form: species and speciation (pp. 57–75). Oxford University Press, New York.
de Queiroz, K. 2007. Species concepts and species delimitation. Systematic Biology, 56(6), 879–886. https://doi.org/10.1080/10635150701701083
de Laubenfels, D. J. & F. Adema. 1998. A taxonomic revision of the genera Cycas and Epicycas gen. nov. (Cycadaceae). Blumea, 43, 351–400.
Degnan, J. L. & N. A. Rosenberg. 2009. Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends in Ecology and Evolution, 24(6), 332–340. https://doi.org/10.1016/j.tree.2009.01.009
DeSalle, R., M. G. Egan & M. Siddall, M. 2005. The unholy trinity: Taxonomy, species delimitation and DNA barcoding. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 360(1462), 1905–1916. https://doi.org/10.1098/rstb.2005.1722
Dohzhansky, T. 1935. A critique of the species concept in biology. Philosophy of Science, 2, 344–355.
Donaldson, J. S. 2003. Cycads: Status Survey and Conservation Action Plan. IUCN/SSC Cycad Specialist Group, IUCN, Gland, Switzerland and Cambridge, UK.
Feng, X., Y. Zheng & X. Gong. 2016. Middle-Upper Pleistocene climate changes shaped the divergence and demography of Cycas guizhouensis (Cycadaceae): evidence from DNA sequences and microsatellite markers. Scientific Reports, (6), 27368. https://doi.org/10.1038/srep27368
Franz, N. M., R. K. Peet & A. S. Weakley. 2008. On the use of taxonomic concepts in support of biodiversity research and taxonomy. In Q. D. Wheeler (Ed.), The New Taxonomy (pp. 63–86). CRC Press.
Goldstein, P. Z. & R. DeSalle. 2011. Integrating DNA barcode data and taxonomic practice: determination, discovery, and description. BioEssays, 33(2), 135–147. https://doi.org/10.1002/bies.201000036
Goloboff, P. A. 2003. Parsimony, likelihood, and simplicity. Cladistics, 19, 91–103. https://doi.org/10.1016/S0748-3007(03)00017-3
González-Astorga, J., A. P. Vovides & C. Iglesias. 2003. Morphological and geographic variation of the cycad Dioon edule Lindl. (Zamiaceae): ecological and evolutionary implications. Botanical Journal of the Linnean Society, 141(4), 465–470. https://doi.org/10.1046/j.1095-8339.2003.00155.x
Grace, O. M., O. A. Pérez-Escobar, E. J. Lucas, M. S. Vorontsova, G. P. Lewis, B. E. Walker, L. G. Lohmann, S. Knapp, P. Wilkie, T. Sarkinen, I. Darbyshire, E. N. Lughadha, A. Monro, Y. Woudstra, S. Demissew, A. M. Muasya, S. Díaz, W. J. Baker & A. Antonelli. 2021. Botanical Monography in the Anthropocene. Trends in Plant Science, 26, 433–441. https://doi.org/10.1016/j.tplants.2020.12.018
Gutiérrez-Ortega, J. S., M. M. Salinas-Rodríguez, J. F. Martínez, F. Molina-Freaner, M. A. Pérez-Farrera, A. P. Vovides, Y. Matsuki, Y. Suyama, T. A Ohsawa, Y. Watano & T. Kajita. 2018a. The phylogeography of the cycad genus Dioon (Zamiaceae) clarifies its Cenozoic expansion and diversification in the Mexican transition zone. Annals of Botany, 121, 535–548. https://doi.org/10.1093/aob/mcx165.
Gutiérrez-Ortega, J. S., T. Yamamoto, A. P. Vovides, M. A. Pérez-Farrera, J. F. Martínez, F. Molina-Freaner, Y. Watano & T. Kajita. 2018b. Aridification as a driver of biodiversity: a case study for the cycad genus Dioon (Zamiaceae). Annals of Botany, 121, 47–60. https://doi.org/10.1093/aob/mcx123
Gutiérrez-Ortega, J. S., M. M. Salinas-Rodríguez, T. Ito, M. A. Pérez-Farrera, A. P. Vovides, J. F. Martínez, F. Molina-Freaner, A. Hernández-López, L. Kawaguchi, A. J. Nagano, T. Kajita, Y. Watano, T. Tsuchimatsu, Y. Takahashi & M. Murakam. 2020a. Niche conservatism promotes speciation in cycads: the case of Dioon merolae (Zamiaceae) in Mexico. New Phytologist, 227(6), 1872–1884. https://doi.org/10.1111/nph.16647
Gutiérrez-Ortega, J. S., M. A. Pérez-Farrera, A. P. Vovides, S. H. Salas-Morales & J. Chemnick. 2020b. Dioon oaxacensis (Zamiaceae): a new cycad species from the arid central valleys of Oaxaca (Mexico). Phytotaxa, 474(1), 51–61. https://doi.org/10.11646/phytotaxa.474.1.5
Gutiérrez-Ortega, J. S., M. A. Pérez-Farrera, A. P. Vovides, A. Chávez-Cortázar, S. López, N. G. Santos-Hernández & S. K. Ruíz-Roblero. 2021. Ceratozamia sanchezae (Zamiaceae): a new cycad species from Chiapas Highlands (Mexico). Phytotaxa, 500(3), 201–216. https://doi.org/10.11646/phytotaxa.500.3.4
Habib, S., Y. Gong, S. Dong, A. Lindstrom, D. W. Stevenson, Y. Liu, W. H. Wu & S. Zhang. 2022. Phylotranscriptomics reveal the spatio-temporal distribution and morphological evolution of Macrozamia, an Australian endemic genus of Cycadales. Annals of Botany, 130, 671–685. https://doi.org/10.1093/aob/mcac117
Hamilton, C. W. & S. H. Reichard. 1992. Current practice in the use of subspecies, variety, and forma in the classification of wild plants. Taxon, 41(3), 485–498. https://doi.org/10.2307/1222819
Haynes, J. L. 2020. Review of “Taxonomic revision of the genus Dioon (Zamiaceae)” published in Phytotaxa 442(4): 267–290. Phytotaxa, 471(1), 69–89. https://doi.org/10.11646/phytotaxa.471.1.8
Hernández-Tapia, J. E., J. Jiménez-Ramírez & A. P. Vovides. 2020. Taxonomic revision of the genus Dioon (Zamiaceae). Phytotaxa, 444(4), 267–290. https://doi.org/10.11646/phytotaxa.442.4.2
Hill, K. D. 1994a. Character evolution, species recognition and classification concepts in the Cycadaceae. In T. Walters & R. Osborne (Eds.), Cycad classification: Concepts and recommendations (pp. 23–44). CABI Publishing, Wallingford, England.
Hill, K. D. 1994b. The Cycas rumphii Complex (Cycadaeeae) in New Guinea and the Western Pacific. Australian Systematic Botany, 7, 543–567. https://doi.org/10.1071/sb9940543
Hill, K. D. 1995a. The genus Cycas in the Indian region, with notes on the application and typification of the name Cycas circinalis. Taxon, 44(1), 23–31. https://doi.org/10.2307/1222674
Hill, K. D. 1995b. Taxonomic changes in the Australian cycads. In E. Vorster (Ed.), Proceedings of the Third International Conference on Cycad Biology (pp. 193–223). Cycad Society of South Africa, Stellenbosch.
Hill, K. D. 1996. A taxonomic revision of the genus Cycas (Cycadaceae) in Australia. Telopea, 7(1), 1–64. https://doi.org/10.7751/telopea19963040
Hill, K. D. 1998. Cycadophyta. In A. Orchard (Ed.), Flora of Australia 48 (pp. 698–661). CSIRO Publishing: Melbourne.
Hill, K. D. 2008. The genus Cycas (Cycadaceae) in China. Telopea, 12(1), 71–118.
Hill, K. D. & S. L. Yang. 1999. The genus Cycas (Cycadaceaei) in Thailand. Brittonia, 51, 48–73. https://doi.org/10.2307/2666557
Hill, K. D., H. T. Nguyen & P. K. Loc. 2004. The genus Cycas (Cyeadaceae) in Vietnam. The Botanical Review, 70(2), 134–193.
Hooker, W. J. 1863. Bowenia spectabilis. Australian Bowenia. Botanical Magazine, 89, Tab. 5398.
Huelsenbeck, J. P., P. Andolfatto & E. T. Huelsenbeck. 2011. Structurama: Bayesian inference of population structure. Evolutionary Bioinformatics, 7, 55–59. https://doi.org/10.4137/EBO.S6761
Johnson, L. A. S. 1959. The Families of cycads and Zamiaceae of Australia. Proceedings of the Linnean Society of New South Wales, 84(1), 64–117.
Johnson, L. A. S. 1961. Zamiaceae. Flora of New South Wales. Contributions from the New South Wales National Herbarium, 1, 21–41.
Jones, K. 1998. Robertsonian fusion and centric fission in karyotype evolution of higher plants. The Botanical Review, 64(3), 273–289.
Knapp, S. 2000. What’s in a name? Linnaeus’ marginal jottings created order out of botanical chaos. Nature, 408, 33. https://doi.org/10.1038/35040663
Knapp, S., G. Lamas, E. N. Lughadha & G. Novarino. 2004. Stability or stasis in the names of organisms: the evolving codes of nomenclature. Philosophical Transactions of the Royal Society B, 359, 611–622. https://doi.org/10.1098/rstb.2003.1445
Knowles, L. L. & B. C. Carstens. 2007. Delimiting species without monophyletic gene trees. Systematic Biology, 56(6), 887–895. https://doi.org/10.1080/10635150701701091
Kotov, A. A. & M. A. Gololobova. 2016. Traditional taxonomy: Quo vadis? Integrative Zoology, 11, 490–495. https://doi.org/10.1111/1749-4877.12215
Laland, K. N., K. Sterelny, J. Odling-Smee, W. Hoppitt & T. Uller. 2011. Cause and effect in biology revisited: is Mayr’s proximate-ultimate dichotomy still useful? Science, 334, 1512–1516. https://doi.org/10.1126/science.121087
Laland, K. N., T. Uller, M. W. Feldman, K. Sterelny, G. B. Müller, A. Moczek, E. Jablonka & J. Odling-Smee. 2015. The extended evolutionary synthesis: its structure, assumptions and predictions. Proceedings of the Royal Society of London B, 282, 20151019. https://doi.org/10.1098/rspb.2015.1019
Landry, G. 1993. Zamiaceae. Flora of North America, 2, 347–349.
Limón, F., J. González-Astorga, F. Nicolalde-Morejón & R. Guevara. 2016. Phenotypic variation of Zamia loddigesii Miq. and Z. prasina W.Bull. (Zamiaceae, Cycadales): the effect of environmental heterogeneity. Plant Systematics and Evolution, 302, 1395–1404. https://doi.org/10.1007/s00606-016-1338-y
Lindstrom, A. J. 2002. Circumscription and lectotypification of C. rumphii. Brittonia, 54, 305–309.
Lindstrom, A. J. & K. D. Hill. 2007. The genus Cycas (Cycadaceae) in India. Telopea, 11(4), 463–488.
Lindstrom, A. J., K. D. Hill & L. C. Stanberg. 2008. The genus Cycas (Cycadaceae) in The Philippines. Telopea, 12(1), 119–145.
Lindstrom, A. J., M. Calonje, D. W. Stevenson, C. Husby & A. Taylor. 2013. Clarification of Zamia acuminata and a new Zamia species from Coclé Province, Panama. Phytotaxa, 98(2), 27–42. https://doi.org/10.11646/phytotaxa.98.2.1
Linnaeus, C. 1763. Palmae pennatifoliae. Species Plantarum, 2, 1658–1659.
Little, D. P. & D. W. Stevenson. 2007. A comparison of algorithms for the identification of specimens using DNA barcodes: examples from gymnosperms. Cladistics, 22, 1–21. https://doi.org/10.1111/j.1096-0031.2006.00126.x.
Liu, J., W. Zhou & X. Gong. 2015. Species delimitation, genetic diversity and population historical dynamics of Cycas diannanensis (Cycadaceae) occurring sympatrically in the Red River region of China. Frontiers in Plant Science, 6, 696. https://doi.org/10.3389/fpls.2015.00696
Liu, J., S. Zhang, N. Nagalingum, Y. C. Chiang, A. Lindstrom & G. Xun. 2018. Phylogeny of the gymnosperm genus Cycas L. (Cycadaceae) as inferred from plastid and nuclear loci based on a large-scale sampling: evolutionary relationships and taxonomical implications. Molecular Phylogenetics and Evolution, 127, 87–97. https://doi.org/10.1016/j.ympev.2018.05.019
Mallet, J. 1995. A species definition for the Modern Synthesis. Trends in Ecology and Evolution, 10(7), 294–298. https://doi.org/10.1016/0169-5347(95)90031-4
Mallet, J. & K. Willmott. 2003. Taxonomy: renaissance or Tower of Babel? Trends in Ecology and Evolution, 18(2), 57–59. https://doi.org/10.1016/S0169-5347(02)00061-7
Marhold, K., T. Stuessy, M. Agababian, D. Agosti, M. H. Alford, A. Crespo, J. V. Crisci, L. J. Dorr, Z. Ferencová, D. Frodin, D. V. Geltman, N. Kilian, H. P. Linder, L. G. Lohmann, C. Oberprieler, L. Penev, G. F. Smith, W. Thomas, M. Tulig, … X. C. Zhang. 2013. The future of botanical monography. Report from an International Workshop, 12–16 March 2012, Smolenice, Slovak Republic. Taxon, 62, 4–20. https://doi.org/10.1002/tax.621003
Martínez-Domínguez, L., F. Nicolalde-Morejón, F. Vergara-Silva & D. W. Stevenson. 2016. Integrative taxonomy of Mexican cycads: biogeography, morphology and DNA barcoding corroborate a new sympatric species in Ceratozamia (Zamiaceae). Phytotaxa, 268(1), 25–45. https://doi.org/10.11646/phytotaxa.268.1.2
Martínez-Domínguez, L., F. Nicolalde-Morejón, F. Vergara-Silva, D. W. Stevenson & E. del Callejo. 2017. Cryptic diversity, sympatry and other integrative taxonomy scenarios in the Mexican Ceratozamia miqueliana complex (Zamiaceae). Organisms, Diversity & Evolution, 17(4), 727–752. https://doi.org/10.1007/s13127-017-0341-7
Martínez-Domínguez, L., F. Nicolalde-Morejón, F. Vergara-Silva & D. W. Stevenson 2018a. Taxonomic review of Ceratozamia (Zamiaceae) in the Sierra Madre Oriental, Mexico. PhytoKeys, 100, 91–124. https://doi.org/10.3897/phytokeys.100.23152
Martínez-Domínguez, L., F. Nicolalde-Morejón, D. W. Stevenson & Q. Santiago-Jiménez. 2018b. Conceptos taxonómicos, fenología y epifitismo: el caso de Ceratozamia tenuis (Zamiaceae). Revista Mexicana de Biodiversidad, 89, 331–339. https://doi.org/10.22201/ib.20078706e.2018.2.2357.
Martínez-Domínguez, L., F. Nicolalde-Morejón, F. G. Lorea-Hernández, F. Vergara-Silva & D. W. Stevenson. 2020. A novelty in Ceratozamia (Zamiaceae, Cycadales) from the Sierra Madre del Sur, Mexico: biogeographic and morphological patterns, DNA barcoding and phenology. PhytoKeys, 156, 1–25. https://doi.org/10.3897/phytokeys.156.53502
Martínez-Domínguez, L., F. Nicolalde-Morejón, F. Vergara-Silva & D. W. Stevenson. 2022. Monograph of Ceratozamia (Zamiaceae, Cycadales): an endangered genus. PhytoKeys, 208, 1–102. https://doi.org/10.3897/phytokeys.208.80382
Mayden, R. 1999. Consilience and a hierarchy of species concepts: advances toward closure on the species puzzle. Journal of Nematology, 31(2), 95–116.
McVaugh, R. & J. A. Pérez de la Rosa. 1992. Zamiaceae. In R. McVaugh (Ed.), Flora Novo-Galiciana. A descriptive account of the vascular plants of western Mexico (pp. 107–119). The University of Michigan Herbarium ann arbor.
McVaugh, R. 1992. Flora Novo-Galiciana: a descriptive account of the vascular plants of Western Mexico, Gymnosperms and Pteridophytes, 17, 107–119.
Medina, R. & P. Dávila. 1997. Flora del Valle de Tehuacán-Cuicatlán, 12, 20–24.
Medina-Villarreal, A. & J. González-Astorga. 2016. Morphometric and geographical variation in the Ceratozamia mexicana Brongn. (Zamiaceae) complex: evolutionary and taxonomic implications. Biological Journal of the Linnean Society, 119(1), 213–233. https://doi.org/10.1111/bij.12806
Medina-Villarreal, A., J. González-Astorga & A. Espinosa de los Monteros. 2019. Evolution of Ceratozamia cycads: a proximate-ultimate approach. Molecular Phylogenetics and Evolution, 139, 106530. https://doi.org/10.1016/j.ympev.2019.106530
Meier, R. & R. Willmann. 2000. The hennigian species concept. In Q. Wheeler & R. Meier (Eds.) Species concepts and phylogenetic theory (pp.30–43). New York: Columbia University Press.
Miquel, F. A. W. 1843. De Cycadeis loddigesianis. Tijdschr. Natuurl. Gesch. Physiol. 10, 68–74.
Miquel, F. A. W. 1847. Over eenige nieuwe of zeldzame Cycadeen in den Hortus Botanicus te Amsterdam. Eerste gedeltee. Tijdschr. Wis- Natuurk. Wetensch. Eerste Kl. Kon. Ned. Inst. Wetensch. 1(1), 33–43.
Miquel, F. A. W. 1848. Over eenige nieuwe of zeldame Cycadeen in den Hortus Botanicus te Amsterdam. Derde gedeelte. Tijschr. Wis-en natuurk. Wet. 1(4), 197–208.
Miquel, F. A. W. 1863. Over de Cycadeen in Nieuw-Holland. Verslagen Meded. Afd. Natuurk. Kon. Akad. Wetensch. 15, 363–377.
Miquel, F. A. W. 1868. Nouveaux materiaux pour erver ala connaissance des Cycadees. Cinquieme parties. Archives Néerlandaises des Sciences Exactes et Naturelles 3(5), 403–427.
Miquel, F. A. W. 1869a. Nouveaux materiaux pour servir à la connaissance des Cycadees. Cinqyieme partie. Adansonia, 9, 169–180.
Miquel, F. A. W. 1869b. Nieuwe bijdragen tot de kennis der Cycadeen. Verslagen en Mededeelingen der Koninklijke Akademie van Wetenschappen, Afdeeling Letterkunde. ser. 2. 3(2), 196–206.
Miquel, F. A. W. 1870. Noveaux matériaux pour servir á la connaissance des Cycadées. Sixieme partie. Revision. Classification. Adansonia, 9, 352–367.
Miquel, F. A. W. 1842. Monographia Cycadearum. Trajecti ad Rhenum/Utrecht. Pp. 32–48, t. 4−5.
Miquel, F. A. W. 1861. Prodromus systematis Cycadearum. In honorem festi diei XV kal. m. Julii MDCCCLXI, quo academia rheno-trajectina exacta XLV lustra celebrat. Utrecht, C. van der. Post Jr., Amsterdam, C. G. van der Post.
Mishler, B. D. 2021. What, if anything, are species. CRC Press, Boca Raton, Florida.
Moretti, A. & S. Sabato. 1984. Karyotype evolution by centromeric fission in Zamia (Cycadeles). Plant Systematics and Evolution, 146, 215–233.
Mudannayake, A., L. Ranaweera, P. Samaraweera, S. Sooriyapathirana & A. Perera. 2019. The morpho-genetic and ecological niche analyses reveal the existence of climatically restricted Cycas zeylanica complex in Sri Lanka. Scientific Reports, 9, 16807. https://doi.org/10.1038/s41598-019-53011-w
Mueller, F. J. H. 1858. Notes on some rare and medicinal plants of Australia. Quarterly Journal & Transactions of the Pharmaceutical Society of Victoria, 2, 44–46.
Mueller, F. J. H. 1859. Notes on some rare and medicinal plants of Australia. Quarterly Journal & Transactions of the Pharmaceutical Society of Victoria, 2, 90–91
Muñoz-Rodríguez, P., T. Carruthers, J. R. I. Wood, B. R. M. Williams, K. Weitemier, B. Kronmiller, C. Goodwin, A. Sumadijaya, N. L. Anglin, D. Filer, D. Harris, M. D. Rausher, S. Kelly, A. Liston & R. W. Scotland. 2019. A taxonomic monograph of Ipomoea integrated across phylogenetic scales. Nature plants, 5, 1136–1154. https://doi.org/10.1038/s41477-019-0535-4
Nagalingum, N. S., C. R. Marshall, T. B. Quental, H. S. Rai, D. P. Little & S. Mathews. 2011. Recent synchronous radiation of a living fossil. Science, 334, 796–799. https://doi.org/10.1126/science.1209926
Naomi, S. I. 2011. On the integrated frameworks of species concepts: Mayden’s hierarchy of species concepts and de Queiroz’s unified concept of species. Journal of Zoological Systematics and Evolutionary Research, 49(3), 177–184. https://doi.org/10.1111/j.1439-0469.2011.00618.x
Nicolalde-Morejón, F., A. P. Vovides, D. W. Stevenson & V. Sosa. 2008. The identity of Zamia katzeriana and Z. verschaffeltii (Zamiaceae). Brittonia, 60(1), 38–48.
Nicolalde-Morejón, F., A. P. Vovides & D. W. Stevenson. 2009a. Taxonomic revision of Zamia in Mega-Mexico. Brittonia, 61, 301–335. https://doi.org/10.1007/s12228-009-9077-9
Nicolalde-Morejón, F., F. Vergara-Silva, J. González-Astorga, A. P. Vovides & A. Espinosa de los Monteros, A. 2009b. Reciprocal illumination of morphological characters upon a molecular hypothesis supports the proposal of a new species of cycad from Mexico. Systematics and Biodiversity, 7(1), 73–79. https://doi.org/10.1017/S1477200008002879
Nicolalde-Morejón, F., L. Martínez-Domínguez, D. W. Stevenson & F. Vergara-Silva. 2019. Disentangling the identity of Zamia from Mexican Pacific seaboard, with a description of a new species. Nordic Journal of Botany, 37(9), 1–9. https://doi.org/10.1111/njb.02430
Norstog, K. J. & T. J. Nicholls. 1997. The Biology of the Cycads. Cornell University Press, Ithaca, USA.
Norstog, K. J. & T. J. Nicholls. 1999. Some comments on the taxonomy and systematics of cycads. In C. J. Chen (Ed.), Proceedings of the Fourth International Conference on Cycad Biology (Panzhihua, China) (pp. 92–97). International Academic Publishers.
Olson, K. & R. Gorelick. 2011. Chromosomal fission accounts for small-scale radiations in Zamia (Zamiaceae; Cycadales). Botanical Journal of the Linnean Society, 165, 168–185. https://doi.org/10.1111/j.1095-8339.2010.01102.x
Pérez-Farrera, M. A., A. P. Vovides & C. Iglesias. 1999. A new species of Ceratozamia (Zamiaceae, Cycadales) from Chiapas, Mexico. Novon, 9, 410–413. https://doi.org/10.2307/3391741
Pérez-Farrera, M. A., A. P. Vovides, R. Martínez-Camilo, N. Martínez-Meléndez & C. Iglesias. 2009. A reassessment of the Ceratozamia miqueliana species complex (Zamiaceae) of southeastern Mexico, with comments on species relationships. Systematics and Biodiversity, 7(4), 433–443. https://doi.org/10.1017/S1477200009990211
Pérez-Farrera, M. A., A. P. Vovides, C. Ruíz-Castillejos, S. Galicia, A. Cibrián-Jaramillo & S. López. 2016. Anatomy and morphology suggest a hybrid origin of Zamia katzeriana (Zamiaceae). Phytotaxa, 270(3), 161–181. https://doi.org/10.11646/phytotaxa.270.3.1
Pérez-Farrera, M. A., A. P. Vovides, D. González, S. López, L. Hernandez-Sandoval & M. Martínez. 2017. Estimation of genetic variation in closely related cycad species in Ceratozamia (Zamiaceae: Cycadales) using RAPDs markers. Revista de Biología Tropical, 65(1), 305–319. https://doi.org/10.15517/rbt.v65i1.15266
Pérez-Farrera, M. A., J. S. Gutiérrez-Ortega, J. L. Haynes, J. Chemnick, S. H. Salas-Morales, M. Calonje & A. P. Vovides. 2021. Ceratozamia aurantiaca (Zamiaceae): A new cycad species from the Northern Rainforests of Oaxaca, Mexico. Taxonomy, 1, 243–255. https://doi.org/10.3390/taxonomy1030018
Prata, E. M. B., C. Sass, D. P. Rodrigues, F. M. C. B. Domingos, C. D. Specht, G. Damasco, C. C. Ribas, P. V. A. Fine & A. Vicentini. 2018. Towards integrative taxonomy in Neotropical botany: disentangling the Pagamea guianensis species complex (Rubiaceae). Botanical Journal of the Linnean Society, 188, 213–231. https://doi.org/10.1093/botlinnean/boy051
Radha, P. & R. Singh. 2011. Amplified fragment length polymorphism (AFLP) studies on Indian Cycas species. African Journal of Biotechnology, 10(34), 6381–6386. https://doi.org/10.5897/AJB10.2314
Read, R. W. & M. L. Solt. 1986. Bibliography of the living Cycadales. Lyonia, 2(4), 33–200.
Regel, E. 1857a. Zwei neue Cycadean, die im Botanischen Garten zu Petersburg kultivirt warden, nebst Beiträgen zur Kenntniss dieser Familie. Bulletin de la Société des Naturalistes de Moscou, 30, 163–191.
Regel, E. 1857b. Die Cycadean des botanischen gartens in Petersburg. Gartenflora, 6, 5–16.
Regel, E. 1875. Encephalartos Verschaffelti Rgl.; die Cycadeen unserer Gärten. Gartenflora, 24, 35–43.
Regel, E. 1876a. Die Cycadeen, deren Gattungen und Arten. Gartenflora, 25, 259–262.
Regel, E. 1876b. Cycadearum generum specierumque revisio. Acta Horti Petropolitani, 4(4), 273–320.
Rieppel, O. 2009. Species as a Process. Acta Biotheoretica, 57, 33–49. https://doi.org/10.1007/s10441-008-9057-6.
Sae-Eung, C., T. Kanchanaketu, N. Sangduen & V. Hongtrakul. 2012. DNA methylation and genetic diversity analysis of genus Cycas in Thailand. African Journal of Biotechnology, 11(4), 743–751. https://doi.org/10.5897/AJB11.2835
Saitou, N. & M. Nei. 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4(4), 406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454.
Salzman, S., D. Crook, M. Calonje, D. W. Stevenson, N. E. Pierce & R. Hopkins. 2021. Cycad-weevil pollination symbiosis is characterized by rapidly evolving and highly specific plant-insect chemical communication. Frontiers in Plant Science, 12, 639368. https://doi.org/10.3389/fpls.2021.639368
Schoener, T. W. 1968. Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology, 49, 704–726. https://doi.org/10.2307/1935534
Schuster, J. 1932. Cycadaceae. In Engler, A. (Ed.), Das Pflanzenreich, 99, 1−168.
Schutzman, B. 2004. Systematics of Meso-American Zamia (Zamiaceae). In T. Walters, & R. Osborne. (Eds.), Cycad classification: concepts and recommendations (pp. 159–172). CABI Publising. UK.
Segalla, R., R. Soares-Pimenta & M. Calonje. 2023. Zamia multidentata (Cycadales, Zamiaceae), a new arborescent species of Zamia from Acre, Brazil. Phytotaxa, 598, 21−31.
Sharma, I. K., D. L. Jones, P. I. Forster & A. G. Young. 1998. The extent and structure of genetic variation in the Macrozamia pauli–guilielmi complex (Zamiaceae). Biochemical Systematics and Ecology, 26, 45–54.
Sigwart, J. D. 2019. What species mean: a users guide to the units of biodiversity. CRC Press.
Sites J. W. & J. Marshall. 2003. Delimiting species: a renaissance issue in systematic biology. Trends in Ecology and Evolution, 18, 462−470. https://doi.org/10.1016/S0169-5347(03)00184-8
Sites J. W. & J. Marshall. 2004. Operational criteria for delimiting species. Annual Review of Ecology, Evolution, and Systematics, 35, 199–227. https://doi.org/10.1146/annurev.ecolsys.35.112202.130128
Slater, M. H. 2016. An essay on the metaphysics of species. Palgrave Macmillian. Basinstoke, New York.
Sneath, P. H. A. 1976. Phenetic taxonomy at the species level and above. Taxon, 25(4), 437–450. https://doi.org/10.2307/1220526
Sokal, R. R. & T. J. Crovello. 1970. The biological species concept: a critical evaluation. The American Naturalist, 104, 127–153.
Stevenson, D. W. 1991a. The Zamiaceae in the Southeastern United States. J. Arnold Arb. Suppl. Series, 1, 367–383.
Stevenson, D. W. 1991b. Cycadaceae. Flora of the Guianas. Ser. A, Fasc. 9, 3–6.
Stevenson, D. W. 1991c. Zamiaceae. Flora of the Guianas. Ser. A, Fasc. 9, 7–11.
Stevenson, D. W. 1993. The Zamiaceae in Panama with comments on phytogeography and species relationships. Brittonia, 45(1), 1–16. https://doi.org/10.2307/2806850
Stevenson, D. W. 2001. Orden Cycadales. Flora de Colombia, 21, 1–91.
Stevenson, D. W. 2004. Zamiaceae of Bolivia, Ecuador, and Peru. In T. Walters & Osborne, R. (Eds.), Cycad Classification: Concepts and Recommendations (pp. 173–194). CABI Publishing, Oxford.
Stevenson, D. W. 2006. Zamiaceae. Flora of the Venezuelan Guayana. 9, 575–576.
Stevenson, D. W. & S. Sabato. 1986a. Typification of names in Zamia and Aulacophyllum (Zamiaceae). Taxon, 35(1), 134–144. https://doi.org/10.2307/1221051
Stevenson, D. W. & S. Sabato. 1986b. Typification of names in Ceratozamia, Dion, and Microcycas (Zamiaceae). Taxon, 35(3), 578–584. https://doi.org/10.2307/1221921
Stevenson, D. W., S. Sabato & M. Vázquez-Torres. 1986. A new species of Ceratozamia (Zamiaceae) from Veracruz, Mexico with comments on species relationships, habitats, and vegetative morphology in Ceratozamia. Brittonia, 38(1), 17–26. https://doi.org/10.2307/2807413
Stull, G. W., X. J. Qu, C. Parins-Fukuchi, Y. Y. Yang, J. B. Yang, Z. Y. Yang, Y. Hu, H. Ma, P. S. Soltis, D. E. Soltis, D. Z. Li, S. A. Smith & T. Yi. 2021. Gene duplications and phylogenomic conflict underlie major pulses of phenotypic evolution in gymnosperms. Nature Plants, 7, 1015–1025 https://doi.org/10.1038/s41477-021-00964-4
Thiselton-Dyer, W. T. T. 1884. Order CXXXVII. Cycadaceae. Biologia Centrali-Americana Botany, 3, 190–195.
Turland, N. J., J. H. Wiersema, F. R. Barrie, W. Greuter, D. L. Hawksworth, P. S. Herendeen, S. Knapp, W. H. Kusber, D. Z. Li, K. Marhold, T. W. May, J. McNeill, A. M. Monro, J. Prado, M. J. Price & G. F. Smith. (Eds.). 2018. International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Glashütten: Koeltz Botanical Books.
Valdecasas, A. G., M. L. Pelaez & Q. D. Wheeler. 2014. What’s in a (biological) name? The wrath of Lord Rutherford. Cladistics, 30, 215−223. https://doi.org/10.1111/cla.12035
Van Valen, L. 1976. Ecological species, multispecies, and oaks. Taxon, 25, 233–239. https://doi.org/10.2307/1219444
Vorster, P. J. 2004. Typification of Encephalartos. Bothalia, 34(2), 112–113.
Vovides, A. P. 1999. Flora del Bajío y de Regiones Adyacentes 71, 1–16.
Vovides, A. P., M. A. Pérez-Farrera, D. González & S. Avendaño. 2004. Relations and phytogeography in Ceratozamia (Zamiaceae). In T. Walters, & Osborne, R. (Eds.), Cycad classification: concepts and recommendations (pp. 109–125). CABI Publishing. UK.
Vovides, A. P., S. Avendaño, M. A. Pérez-Farrera & D. W. Stevenson. 2012. What is Ceratozamia brevifrons (Zamiaceae)? Brittonia, 64(1), 35–42. https://doi.org/10.1007/s12228-011-9199-8
Vovides, A. P., D. W. Stevenson, M. A. Pérez-Farrera, S. López & S. Avendaño. 2016. What is Ceratozamia mexicana (Zamiaceae)? Botanical Sciences, 94 (2), 419–429. https://doi.org/10.17129/botsci.449
Vovides, A. P., M. A. Pérez-Farrera, J. S. Gutiérrez-Ortega, S. Avendaño, A. Medina-Villarreal, J. González-Astorga & S. Galicia. 2020. A revision of the Ceratozamia miqueliana (Zamiaceae) species complex based on analyses of leaflet anatomical characters. Flora, 270, 151649. https://doi.org/10.1016/j.flora.2020.151649
Vovides, A. P., J. D. Rees & M. Vázquez-Torres. 1983. Zamiaceae. Flora de Veracruz. INIREB.
Wake, D. B., M. H. Wake & C. D. Specht. 2011. Homoplasy: from detecting pattern to determining process and mechanism of evolution. Science, 331, 1032–1035. https://doi.org/10.1126/science.1188545
Wang, D. Y. 1996. Systematic classification and taxonomy. In F. X. Wang, & Liang, H. B. (Eds.), Cycads in China (pp. 9–142). Guangdong Science and Technology Press: Guangdong.
Wang, X. H., W. Wu, S. G. Jian. 2019. Transcriptome analysis of two radiated Cycas species and the subsequent species delimitation of the Cycas taiwaniana complex. Applications in Plant Sciences, 7, E11292. https://doi.org/10.1002/aps3.v7.10.10.1002/aps3.11292
Warburg, O. 1900. Cycadaceae. Monsunia 1, 178–181.
Warren, D. L., R. E. Glor & M. Turelli. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution, 62(11), 2868–2883. https://doi.org/10.1111/j.1558-5646.2008.00482.x
Wheeler, Q. D. 2004. Taxonomic triage and the poverty of phylogeny. Philosophical Transactions of the Royal Society of London B, 359, 571–583. https://doi.org/10.1098/rstb.2003.1452
Wheeler, Q. D. 2023. Species, Science and Society: The Role of Systematic Biology. London: Routledge.
Wheeler, Q. D. & N. I. Platnick. 2000. The phylogenetic species concept. In Q. D. Wheeler, & R. Meier (Eds.), Species concepts and phylogenetic theory: a debate (pp. 55–69). New York: Columbia Univ. Press.
Wheeler, Q. D., P. H. Raven & E. O. Wilson. 2004. Taxonomy: impediment or expedient? Science, 303(5656), 285. https://doi.org/10.1126/science.303.5656.285
Wheeler, Q. D., S. Knapp, D. W. Stevenson, J. Stevenson, S. D. Blum , B. M. Boom , G. G. Borisy , J. L. Buizer , M. R. De Carvalho, A. Cibrian , M. J. Donoghue , V. Doyle , E. M. Gerson , C. H. Graham , P. Graves , S. J. Graves , R. P. Guralnick , A. L. Hamilton , J. Hanken,… J. B. Wooley. 2012. Mapping the biosphere: exploring species to understand the origin, organization and sustainability of biodiversity. Systematics and Biodiversity, 10(1), 1–20. https://doi.org/10.1080/14772000.2012.665095
Whitelock, L. M. 2004. Classification concepts in Ceratozamia (Zamiaceae). In T. Walters, & R. Osborne (Eds.), Cycad classification: concepts and recommendations (pp. 95–108). CABI Publishing.
Wiens, J. J. 2007. Species delimitation: new approaches for discovering diversity. Systematic Biology, 56, 875–878. https://doi.org/10.1080/10635150701748506
Wiens, J. J. & T. A. Penkrot. 2002. Delimiting species using DNA and morphological variation and discordant species limits in spiny lizards (Sceloporus). Systematic Biology, 51, 69–91. https://doi.org/10.1080/106351502753475880
Wiley, E. O. & R. L. Mayden. 2000. The evolutionary species concept. In Q. D. Wheeler, & R. Meier (Eds.), Species concepts and phylogenetic theory: a debate (pp. 70–89). New York: Columbia Univ. Press.
Wilkins, J. S. 2023. Understanding Species. Cambridge University Press, Cambridge. 160 pp.
Xiao, L. Q. & X. Gong. 2006. Genetic differentiation and relationships of populations in the Cycas balansae complex (Cycadaceae) and its conservation implications. Annals of Botany, 97(5), 807–812. https://doi.org/10.1093/aob/mcl039
Zachos, F. E. 2016a. A brief history of species concepts and the species problem. In Species concepts in biology: historical development, theoretical foundations and practical relevance (pp. 17–44). Springer International Publishing, Cham, Switzerland.
Zachos, F. E. 2016b. Species concepts and beyond: selected topics relating to the species problem. In Species concepts in biology: historical development, theoretical foundations and practical relevance (pp. 97–137). Springer International Publishing, Cham, Switzerland.
Zachos, F. E. 2016c. The practical relevance of species concepts and the species problem. In Species concepts in biology: historical development, theoretical foundations and practical relevance (pp. 163–174). Springer International Publishing, Cham, Switzerland.
Zhang, Z. Q. 2020. Promoting excellence of monographs in taxonomy. Megataxa, 001(2), 141–142. https://doi.org/10.11646/megataxa.1.2.4
Zhou, W., M. M. Guan & X. Gong. 2015. Cycas chenii (Cycadaceae), a new species from China, and its phylogenetic position. Journal of systematics and evolution, 53(6), 489–498. https://doi.org/10.1111/jse.12153
Acknowledgements
The first author thanks Posgrado en Ciencias Biológicas (UNAM) and Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) for the doctoral fellowship (729810). This work is part of the requirements at the Posgrado en Ciencias Biológicas-UNAM for L. Martínez-Domínguez to obtaining her PhD degree. We are thankful to María Hilda Flores Olvera for advice and helpful comments on taxonomic concepts and taxonomy. Also, we thank Nidia Mendoza Díaz for discussions of species concepts. We thank The World List Cycads for providing some photos of strobili, which were edited for our assemblage in Figure 3. This research received support in part by Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) grant 134960 to FNM, an IAPT Research Grants 2020 to LMD, and National Science Foundation (NSF) Grants BSR-8607049, EF-0629817 and DEB-2140319 to DWS.
Funding
This research was financed in part by CONACyT grant 134960 to FNM, an IAPT Research Grants to LMD, and NSF Grants BSR-8607049, EF-0629817 and DEB-2140319 to DWS.
Author information
Authors and Affiliations
Contributions
LMD conceived and initiated the research, performed the literature search, data analysis, and wrote the first draft of the paper. DWS contributed with the compilation of original papers (historical taxonomic treatments) for the database. FNM, DWS and FVS drafted and critically revised the work.
Corresponding author
Ethics declarations
Disclosure of Potential Conflicts of Interest
D. Wm. Stevenson is member of the Editorial Board of The Botanical Review. LMD, FNM and FVS declare that they have no conflicts of interest.
Research involving Human Participants and/or Animals
This statement is not applicable.
Informed Consent
This statement is not applicable.
Financial Interests
The authors declare they have no financial interests.
Competing Interests
D. Wm. Stevenson is member of the Editorial Board of The Botanical Review. This research received support in part by CONACyT grant 134960 to FNM, and NSF Grants BSR-8607049, EF-0629817 and DEB-2140319 to DWS.
Supplementary Information
Below is the link to the electronic supplementary material.
12229_2023_9293_MOESM3_ESM.jpg
Supplementary file3 (JPG 2024 KB) Supporting Information Figure S3. Total cumulative number of scientific names published each year in (a) Bowenia, (b) Ceratozamia, (c) Cycas, (d) Dioon, (e) Encephalartos and (f) Lepidozamia, (g) Macrozamia, (h) Microcycas, (i) Stangeria and (j) Zamia, from 1753 up to September 2022.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez-Domínguez, L., Nicolalde-Morejón, F., Vergara-Silva, F. et al. A Review of Taxonomic Concepts and Species Delimitation in Cycadales. Bot. Rev. 90, 33–66 (2024). https://doi.org/10.1007/s12229-023-09293-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-023-09293-x