Abstract
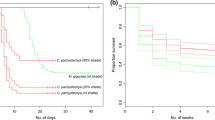
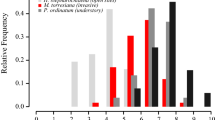
Plants growing in the same environment typically share ecophysiological characteristics enabling them to survive and reproduce when exposed to a common selection pressure. As such, seedlings establishing in temperate forest habitats cope with similar issues of low light availability and climate seasonality. We examined similarities in the germination syndromes in three shade-tolerant temperate forest herbs, Allium ursinum (Amaryllidaceae), Mercurialis perennis (Euphorbiaceae) and Dioscorea communis (Dioscoreaceae) with a distant phylogenetic affiliation. Seeds of M. perennis and A. ursinum are dispersed in spring and those of D. communis in autumn. Experiments on phenology of seed germination and seedling emergence in natural conditions revealed that seeds of all three species germinate in the following autumn. Seedlings do not emerge immediately but rather remain below the soil surface until late winter or early spring. Experiments in controlled laboratory conditions showed that seeds germinated best at intermediate autumn or spring temperatures (between 10 and 15°C) following a period at high summer temperatures (around 20°C). Seed germination of Allium ursinum is strongly photoinhibited, while moderate photoinhibition and no photoinhibition at all was observed in Dioscorea communis and Mercurialis perennis, respectively. Although seeds of all three species are endospermic at dispersal, no embryo growth prior to germination was observed. The cotyledons functioned as a haustorium, recuperating the nutrient reserves in the endosperm post-germination. It can be concluded that although phylogenetically unrelated, the three species studied show a remarkable similarity in germination and seedling emergence strategy that is commonly observed in plants adapted to growing in shady conditions.



Similar content being viewed by others
References
Angevine MW, Chabot BF (1979) Seed germination syndromes in higher plants. In Solbrig OT, Jain S, Johnson GB, Raven PH (eds) Topics in plant population biology. Palgrave, London, pp 188–206
Barton LV (1933) Seedling production in tree peony. Contr Boyce Thompson Inst Pl Res 5:451–460
Barton LV, Schroeder, EM (1942) Dormancy in seeds of Convallaria majalis L. and Smilacina racemosa. Contr Boyce Thompson Inst Pl Res 12:277–300
Baskin JM, Baskin CC (1985) Epicotyl dormancy in seeds of Cimicifuga racemosa and Hepatica acutiloba. Bull Torrey Bot Club 112:253–257
Baskin CC, Baskin JM (1988) Germination ecophysiology of herbaceous plant species in a temperate region. Amer J Bot 75:286–305
Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Sci Res 14:1–16
Baskin CC, Baskin JM (2014) Seeds. Ecology, biogeography, and evolution. 2nd Ed. Academic Press, San Diego
Bierzychudek P (1982) Life histories and demography of shade-tolerant temperate forest herbs: a review. New Phytol 90:757–776
Burkill IH (1939) The trigger-mechanism in the germination of the seed of Tamus communis Linn. J Bot 77:44–50
Burkill IH (1944) Biological flora of the British Isles. Tamus communis J Ecol 32:121–129
Carta A, Probert R, Moretti M, Peruzzi L, Bedini, G (2014) Seed dormancy and germination in three Crocus ser. verni species (Iridaceae): implications for evolution of dormancy within the genus. Pl Biol 16:1065–1074
Carta A, Hanson S, Müller JV (2016) Plant regeneration from seeds responds to phylogenetic relatedness and local adaptation in Mediterranean Romulea (Iridaceae) species. Ecol Evol 6:4166–4178
Carta A, Skourti E, Mattana E, Vandelook F, Thanos CA (2017) Photoinhibition of seed germination: occurrence, ecology and phylogeny. Seed Sci Res 27:131–153
Commander LE, Merritt DJ, Rokich DP, Dixon KW (2009) The role of after-ripening in promoting germination of arid zone seeds: a study on six Australian species. Bot J Linn Soc 161:411–421
Copete E, Herranz JM, Ferrandis P, Baskin CC, Baskin JM (2011) Physiology, morphology and phenology of seed dormancy break and germination in the endemic Iberian species Narcissus hispanicus (Amaryllidaceae). Ann Bot (Oxford) 107:1003–1016
Crocker W (1916) Mechanics of dormancy in seeds. Amer J Bot 3:99–120
Ensslin A, Van de Vyver A, Vanderborght T, Godefroid S (2018). Ex situ cultivation entails high risk of seed dormancy loss on short-lived wild plant species. J Appl Ecol 55:1145–1154
Ernst WHO (1979) Population biology of Allium ursinum in northern Germany. J Ecol 67:347–362
Findeis M (1917) Über das Wachstum des Embryos im ausgesäeten Samen vor der Keimung. Sitzungsber Akad Wiss Math-Naturwiss Kl 126:77–102
Fox JF (1982) Adaptation of gray squirrel behavior to autumn germination by white oak acorns. Evolution 36:800–809
Hermy M, Honnay O, Firbank L, Grashof-Bokdam C, Lawesson JE (1999) An ecological comparison between ancient and other forest plant species of Europe, and the implications for forest conservation. Biol Conservation 91:9–22
Hodgson JG, Mackey JML (1986) The ecological specialization of dicotyledonous families within a local flora: some factors constraining optimization of seed size and their possible evolutionary significance. New Phytol 104:497–515
Hoffman CA (1933) Developmental morphology of Allium cepa. Bot Gaz 95:279–299
Jankowska-Blaszczuk M, Daws MI (2007) Impact of red: far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil. Funct Ecol 21:1055–1062
Jefferson RG (2008) Biological Flora of the British Isles: Mercurialis perennis L. J Ecol 96:386–412
Klimešová J, Martínková J, Ottaviani G (2018) Belowground plant functional ecology: towards an integrated perspective. Funct Ecol 32:2115–2126
Kondo T, Narita M, Phartyal SS, Hidayati SN, Walck JL, Baskin JM, Baskin CC (2015) Morphophysiological dormancy in seeds of Convallaria keiskei and a proposal to recognize two types of double dormancy in seed dormancy classification. Seed Sci Res 25:210–220
Krähenbühl M, Yuan YM, Küpfer P (2002). Chromosome and breeding system evolution of the genus Mercurialis (Euphorbiaceae): implications of ITS molecular phylogeny. Pl Syst Evol 234:155–169
Lakon 1911 Beiträge zur forstlichen Samenkunde. II. Zur Anatomie und Keimungsphysiologie der Eschensamen. Naturwoss Z Forst- Landw 9:285
Lawton JRS, Lawton JR (1967) The morphology of the dormant embryo and young seedling of five species of Dioscorea from Nigeria. Proc Linn Soc London 178:153–159
Li QQ, Zhou SD, He XJ, Yu Y, Zhang YC, Wei XQ (2010) Phylogeny and biogeography of Allium (Amaryllidaceae: Allieae) based on nuclear ribosomal internal transcribed spacer and chloroplast rps16 sequences, focusing on the inclusion of species endemic to China. Ann Bot (Oxford) 106:709–733
Magyar L, Lukács D (2002) Germination and emergence of annual mercury (Mercurialis annua L.). Z Pflanzenkrankh Pflanzenschutz 18:197–203
Martin AC (1946) The comparative internal morphology of seeds. Amer Midl Naturalist 36:513–660
Mondoni A, Probert R, Rossi G, Hay F, Bonomi C (2008). Habitat-correlated seed germination behaviour in populations of wood anemone (Anemone nemorosa L.) from northern Italy. Seed Sci Res 18:213–222
Mukerji SK (1936) Contributions to the autecology of Mercurialis perennis L. Parts I–III. J Ecol 24:38–81
Newton RJ, Hay FR, Ellis RH (2013) Seed development and maturation in early spring-flowering Galanthus nivalis and Narcissus pseudonarcissus continues post-shedding with little evidence of maturation in planta. Ann Bot (Oxford) 111:945–955
Okagami N, Kawai M (1977) Dormancy in Dioscorea: gibberellin-induced inhibition or promotion in seed germination of D. tokoro and D. tenuipes in relation to light quality. Pl Physiol 60:360–362
Okagami N, Kawai M (1982) Dormancy in Dioscorea: differences of temperature responses in seed germination among six Japanese species. Bot Mag 95:155–166
R Development Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from http://www.Rproject.org. Accessed 01 June 2018
Slade EA, Causton DR (1979) The germination of some woodland herbaceous species under laboratory conditions: a multifactorial study. New Phytol 83:549–557
Terui K, Okagami N (1989) Dormancy in Dioscorea: rapid germination of detached embryos from dormant seeds of D. tokoro. Pl Cell Physiol 30:287–293
Terui K, Okagami N (1993) Temperature effects on seed germination of East Asian and Tertiary relict species of Dioscorea (Dioscoreaceae). Amer J Bot 80:493–499
Thanos CA, Georghiou K, Douma DJ, Marangaki CJ (1991) Photoinhibition of seed germination in Mediterranean maritime plants. Ann Bot (Oxford) 68:469–475
Thompson K, Hodkinson DJ (1998) Seed mass, habitat and life history: a re-analysis of Salisbury (1942, 1974). New Phytol 138:163–167
Tutin TG (1957) Biological flora of the British Isles. Allium ursinum L. J Ecol 45:1003–1010
Vandelook F, Van Assche JA (2008a). Temperature requirements for seed germination and seedling development determine timing of seedling emergence of three monocotyledonous temperate forest spring geophytes. Ann Bot (Oxford) 102:865–875
Vandelook F, Van Assche JA (2008b). Deep complex morphophysiological dormancy in Sanicula europaea (Apiaceae) fits a recurring pattern of dormancy types in genera with an Arcto-Tertiary distribution. Botany 86:1370–1377
Vandelook F, Janssens SB, Probert RJ (2012) Relative embryo length as an adaptation to habitat and life cycle in Apiaceae. New Phytol 195:479–487
Vandelook F, Newton RJ, Carta A (2018) Photophobia in Lilioid monocots: photoinhibition of seed germination explained by seed traits, habitat adaptation and phylogenetic inertia. Ann Bot (Oxford) 121:405–413
Walck JL, Baskin CC, Baskin JM (2000) Seeds of Thalictrum mirabile (Ranunculaceae) require cold stratification for loss of nondeep simple morphophysiological dormancy. Canad J Bot 77:1769–1776
Whigham DF (2004) Ecology of woodland herbs in temperate deciduous forests. Annual Rev Ecol Evol Syst 35:583–621
Wilkin P, Schols P, Chase MW, Chayamarit K, Furness CA, Huysmans S, Rakotonasolo F, Smets E, Thapyai, C (2005) A plastid gene phylogeny of the yam genus, Dioscorea: roots, fruits and Madagascar. Syst Bot 30:736–749
Wurdack KJ, Hoffmann P, Chase MW (2005) Molecular phylogenetic analysis of uniovulate Euphorbiaceae (Euphorbiaceae sensu stricto) using plastid rbcL and trnL-F DNA sequences. Amer J Bot 92:1397–1420
Acknowledgements
We thank Robin Probert, John Dickie and Rosemary Newton (Royal Botanic Gardens, Kew) for lengthy discussion on notions of the dormancy concept introduced in this manuscript. We thank two anonymous reviewers and the editor for useful suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vandelook, F., Van de Vyver, A. & Carta, A. Three phylogenetically distant shade-tolerant temperate forest herbs have similar seed germination syndromes. Folia Geobot 54, 73–84 (2019). https://doi.org/10.1007/s12224-019-09346-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-019-09346-3