Abstract
In recent years, it is seen that there have been many publications on natural dyes in literature. In the studies carried out, one or more plants were taken and the color and fastness obtained with them in the presence of different mordants were examined. Beyond that, functionalities such as odor, antibacterial activity, and UV protection that natural dyes impart to the fabric were examined. However, the important thing that the literature needs is to represent also the results related to the industrial scale production since industry shows high interest to be able to use natural dyes. In this study, seven plant dyes have been used for the coloration of polyamide fabric. The studies have been conducted both on an industrial scale and at the laboratory scale. Based on the statistical analyses, four dyes with the highest color strength and fastness properties including catechu, madder, mulberry leaf, and pomegranate peel were selected for more studies on industrial scale. The results confirmed the good color strength and satisfactory fastness properties against repeated washing, rubbing, water, and perspiration for all selected dyes. The samples dyed with those dyes exhibited excellent protection against UV radiation. The results obtained in this study showed the potential of the selected natural dyes in eco-friendly industrial dyeing of nylon fabric with acceptable fastness properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Natural colorants are gaining increasing popularity in recent years due to their eco-friendly nature on contrary with the synthetic counterparts which are associated with some health and environmental problems [1,2,3]. Protein fibers such as wool and silk can be easily dyed using various natural dyes, while metal mordants such as alum, ferrous sulfate, potassium dichromate, copper sulfate etc. are generally used for the improvement of their exhaustion and fixation via simultaneous complex formation with the dye and fiber [4, 5].
Natural dyeing of cotton and synthetic fibers is facing more problems due to the low affinity and penetration of natural dyes toward these fibers. As metal mordants are generally considered as pollutants for water and living environment, their use in dyeing processes is restricted [6]. Bio-mordants are natural alternatives for the metal mordants. They contain tannins which are able to form numerous hydrogen bonds with the dye molecules as well as the textile fibers and improve the exhaustion and fastness properties [7]. Ultrasonic irradiation [8], microwave heating [9, 10], plasma pretreatment [11], gamma irradiation [12], chitosan treatment [13], and enzymatic treatment [14] are among the various methods employed for the improvement of natural dyeing of textile fibers.
Nylon is one of the most important synthetic fibers in textile industry which is usually dyed with acid and metal-complex dyes [15]. Attempts have been made to apply natural dyes from various sources in coloration and functionalization of nylon fabrics. Haji et al. [16] applied a natural cationic dye obtained from the roots of Berberis vulgaris on nylon 6 fabric. Atmospheric pressure air plasma and copper mordant were used to improve the dyeability. The fabric dyed under the optimum condition exhibited antibacterial activity against gram-positive and gram-negative bacteria. In another study, when dyeing nylon 6 fabric with indigo, the pretreatment of nylon 6 samples by atmospheric plasma enabled the fabrics to achieve quite high color strength in a shorter time compared with the untreated samples [17]. Bahtiyari and Benli [18] used pomegranate peels and walnut barks for dyeing of polyamide 66 fabric. They employed two different protease enzymes namely trypsin and pepsin, for the pretreatment of the fabrics before natural dyeing at boil. Their results confirmed the successful dyeing of polyamide 66 fabrics with the extracts of pomegranate peels and walnut barks, but the protease enzyme pretreatment showed no significant improvement in the dyeability of the fabrics with any of the used dyes. The wash fastness of the dyed samples was excellent (4–5), while the light fastness was poor (2–3). The studies by Atav and Namirti [19] showed that ozonation of polyamide/Lycra fabric improved the dyeability with walnut rinds and very good fastness properties were obtained.
Bouaziz et al. [20] also showed that nylon fabric can be dyed with pomegranate peels with good washing and rubbing fastness properties, which were further improved using iron sulfate, aluminum sulfate, potassium dichromate, and gallnut as mordants. The light fastness was fair to good and the dyed samples showed antibacterial activity. The highest antibacterial activity was reported in case of sample dyed after mordanting with ferrous sulfate. In another study, extract of white onion skin was applied on nylon fabric using potassium dichromate and ferrous sulfate. The results showed that although the samples dyed without mordanting were dyed with acceptable color strength and fastness properties, post-mordanting using 1%owf of the mordants exhibited the highest color strength and improved fastness properties [21].
Ebrahimi and Parvinzadeh Gashti [22] used henna leave, pomegranate peel, and P. fraxinifolia leaves for natural dyeing of nylon 6 fabric using tannin, tin chloride, and aluminum sulfate as mordants. Their studies confirmed that using tannin resulted in comparable results when it was used as mordant instead of Al and Sn. Kanelli et al. [23] dyed nylon 66 fabric with Violacein, an indole compound produced by Janthinobacterium lividum and obtained the highest color strength when using simultaneous incubation and dyeing. The dyed fabrics showed significant antibacterial and antifungal activities. Jankovic et al. [24] employed 12 pigment-producing Streptomyces strains to dye nylon and nylon/elastane fabrics using living bacterial cultures with satisfactory fastness properties.
Studies of Jankovic et al. [25] showed that dyeing of nylon 66 fabric with cochineal natural dye is an exothermic process following the Nernst isotherm. In another study, three natural flavonoids namely baicalin, quercetin, and rutin were applied on nylon fabric and followed Langmuir–Nernst adsorption isotherm and conformed to the pseudo second-order kinetic model. Quercetin exhibited the highest affinity for the nylon fibers with good fastness properties, and the quercetin-dyed samples showed the highest antioxidant and antibacterial activity compared with the samples dyed with baicalin and rutin [26]. Extracts of faba bean husks and banana peels have been used for natural dyeing of nylon/elastane fabric using various mordants [27, 28]. Myrobalan has been used as a bio-mordant in dyeing of nylon with the extracts of Terminalia arjuna and Thespesia populnea fruits. The yellow dyed samples showed good fastness properties and the color strength was improved when dyeing was done under ultrasonication [29]. Similar results were reported in case of dyeing of nylon with roasted peanut skin as natural dye [30]. Haji et al. [31] applied peppermint, mugworts, Dorema ammoniacum gum, and pomegranate rind as bio-mordants before dyeing of nylon fabric with dragon’s blood resin extract. All bio-mordants improved the color fastness properties while peppermint and artemisia caused the highest improvement in the color strength.
Studies done by Lykidou et al. [32] on curcumin dyeing of wool, cotton, polyamide, and polyester fabrics showed that under the same dyeing condition, nylon was dyed with the highest color strength, exhibiting the highest antibacterial activity and antioxidant properties. The wash and light fastness rating were 5 and 3, respectively. Similarly, the nylon fabrics dyed with the extract of Hawthorn fruits showed good fastness, antibacterial, and antioxidant properties and the dyeing was improved under ultrasonication [33].
Microwave heating and bio-mordants improved the dyeing of nylon with Lac, Cassia obovate, saffron, safflower, and harmala natural dye as well [34,35,36,37]. Eslahi and Maktoobi [38] extracted the keratin from chicken feathers and used it for modification of nylon fabric by exhaustion method. The samples were dyed with sumac as a natural dye and modified fabric showed greater dyeability with high fastness and antibacterial properties. et al. [39] used mahogany (Swietenia mahagoni) seed pod acidic extract (pH 4.5) for optimal dyeing of nylon knit fabric at boil for 60 min. Moderate to good wash and perspiration fastness properties (rating 3–4 and 4, respectively) were achieved while the light fastness was poor (rating 2).
In this study, seven commercially available plant dyes with diverse coloring compound structures have been used for the coloration of polyamide fabric. The studies have been done on laboratory as well as industrial scale. To the best of the knowledge of the authors, there is no significant report on the industrial application of natural dyes on nylon fabrics. Color strength, color coordinates, fastness properties, and UV absorption of each dye on nylon has been evaluated and compared.
2 Experimental
In all trials, ready-to-dye 70/2 denier nylon filament/70 denier gipe nylon interlock (300 g/m2) knitted fabrics were used. All studies within the scope of the study were first carried out in laboratory conditions using soft water at a liquor ratio of 1:10 by exhaust method. Dyeings were carried out in Mathis Labomat laboratory dyeing machine. For this purpose, polyamide fabrics were dyed at 98 °C in 3% owf depth with commercial natural dyes produced from seven different plants. All dyes and auxiliaries used in dyeing processes were kindly supplied from Ama Herbal, Lucknow, India. Common and Latin names of the plants used are given in Table 1.
Before dyeing, fabrics were pre-treated with 5% owf VegeSet (A solution with pH 12 containing beta-carotene and sodium hydroxide) and 5% owf VegePlus (cationization agent for natural dyeing: a solution containing 15% N-acetylglucosamine, 70% diacetyl chitin, and 15% water) at 30 °C for 45 min. After the fabrics were rinsed with cold water, the dyeing process was started. In dyeing processes with madder, a second pre-treatment was done with 10% owf VegeSmart DS (aluminum potassium sulfate dodecahydrate: KAl(SO4)2·12H2O) for 60 min at 30 °C.
Dyeing processes were started at 30 °C and all chemicals (3% owf natural dye, 3% owf VegeDisperse (anionic dispersing agent: solution containing N-acetylglucosamine and water) and 3% owf VegeBiochem [biomordant: rhubarb plant extract with pH between 2 and 4)] were added. Then, the temperature was increased to 98 °C and dyeing was carried out for 45 min at this temperature. In the dyeing process with madder, 3% owf VegeDisperse and soda ash (pH 9) were added to the liquor. After dyeing, the fabric samples were washed twice in cold water and once in hot (80 ℃0 water. In addition to the dyeing made by the aforementioned pre-treatment and using auxiliary chemicals in dyeing, dyeing processes were also carried out without any pre-treatment and without using auxiliary chemicals in the dyebath. The CIE L*a*b* values of the dyed samples were measured with reflectance spectrophotometer. In addition, the colors of the dyed samples were evaluated visually and their photographs were taken under daylight. Statistical analysis of the L* values was made using Minitab 19 program. In dyeing PA fabric with natural dyes, the effect of natural dye type (seven different plants) and chemical usage on the lightness-darkness (L*) of the obtained color was statistically analyzed according to the General Linear Model. Main effects plots were drawn to graphically summarize the results and compare the effects of variables easily.
Since fastness is a factor that is of great importance in terms of dyeing as well as the color obtained, the washing, rubbing, perspiration and light fastness values of the dyed samples were measured. Beyond that, color changes in repeated washings were also examined.
After the laboratory-scale trials were completed, industrial scale trials were also carried out in Gülle Tekstil Inc. (Tekirdağ, Türkiye) with the selected natural dyes. Industrial scale dyeings were also done using soft water at a liquor ratio of 1:10 by exhaust method. Dyeing depth was 3% owf. Dyeings were carried out in a jet dyeing machine (Thies). In industrial scale dyeings only four plant dyes were used which gave best results in laboratory scale experiments. Dyeing processes were started at 30 °C and all chemicals (3% owf natural dye, 3% owf VegeDisperse) were added. Then, the temperature was increased to 98 °C and dyeing was carried out for 45 min at this temperature. In the dyeing process with madder, soda ash (pH 9) was also added to the liquor. After dyeing, the fabric samples were washed twice in cold water and once in hot (80 ℃).
2.1 Color Strength and CIE L*a*b* Values
Datacolor Spectro 1000 spectrophotometer (D 65/10°) was used to determine the reflectance of the dyed samples. Color strength (K/S) values were calculated using the Kubelka–Munk Eq. (1), in which \(R\) is the reflectance value at the wavelength of maximum absorption for each dyed sample.
CIE L*a*b* color coordinates were also measured. The L* represents lightness–darkness component of a dyed sample, with values ranging from 0 (ideal black) to 100 (ideal white). A higher value for L* means a lighter shade. The a* and b* values indicate the dominant hue and chroma of the shade. The positive a* values indicate red while negative a* values correspond with green shade. On the b* axis, positive values correspond with yellow and negative values indicate blue shade. Values closer to 0 mean lower saturation or chroma of the shade. Total color difference value (∆E) was calculated using the following equation [40, 41];
2.2 Fastness Values
The fastness properties of the dyed samples against washing, rubbing (dry and wet), perspiration (acid and alkali), and light were measured according to ISO-105 C06: 2010, ISO 105-X12: 2016, ISO 105-E04: 2013, and ISO 105 B02: 2014, respectively.
2.3 UV Transmission Analysis
UV transmission analysis of fabric samples was performed according to the AATCC 183 standard using an UVTest® Fluorescent/UV Instrument (Atlas). According to this standard, samples with UV protection values below 15 are considered “insufficient”, between 15 and 24 are considered “Good”, samples between 25 and 39 are considered “very good”, samples above 40 are considered “excellent” [42, 43].
3 Results and Discussion
3.1 Results of Laboratory Scale Dyeing
Polyamide fabrics were dyed with seven different natural dyes with and without the use of auxiliary chemicals. The color coordinates and color strength of the dyed samples were measured. The results are given in Table 2. It can be said that the 3% dyeing without the use of auxiliary chemicals before and during dyeing has a lighter shade than the dyeing made using auxiliary chemicals both before and during dyeing. The chemical used in pretreatment before dyeing is a chitosan-based cationization material. Although PA fibers themselves contain amide and amino groups, incorporating additional cationic groups to the fiber before dyeing, increases its affinity for dye molecules. The use of plant-based bio-mordant during dyeing also made the color darker.
Analysis of variance was performed to determine the effect of dye type and chemical usage on the lightness–darkness (L*) value of the color obtained in dyeing, and the results are presented in Table 3. When Table 3 is examined, it is understood that the effect of the natural dye type on the L* value obtained in dyeing is statistically significant (p < 0.05), but the effect of chemical usage is statistically insignificant (p > 0.05). These results reveal that the use of chemicals for natural dyeing, both before and during dyeing, does not provide any additional benefit for polyamide in general. However, when we look at the dye specifically, it can be said that the use of chemicals for marigold, mulberry leaf, pomegranate peel, and myrobalan provides a darker color. Since natural dye type was found to be statistically significant in terms of dyeing efficiency on polyamide, Tukey analyses were also performed to determine the source of the difference and the results are presented in Table 4.
When Table 4 is examined, natural dyes used in the experiments can be divided into three groups according to the ability of dyeing polyamide fibers as follows;
-
- Low yield (Marigold)
-
- Medium yield (Pomegranate peel, rhubarb, mulberry leaf, myrobalan, catechu) and
-
- High yield (Madder)
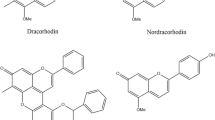
All aforementioned results can be clearly seen from the main effects plot given in Fig. 1. Marigold showed the lowest performance in terms of dyeing efficiency. When the chemical structures of the dyes given in Fig. 2 are examined, it is understood why marigold provides the lowest yield among all plants. When we look at the structure of lutein, which is the main colorant found in marigold, it can be said that it has a rather nonpolar structure compared to the others and the hydroxyl group content, which is effective in forming H bridges with polyamide macromolecules in its structure, remains low compared to its molecular mass. This explains why marigold dyes the polyamide in the lightest color. Considering the potential reason why madder (alizarin) is the substance that dyes the polyamide the darkest among the plants used, it is the smallest molecule (the lowest molecular weight) among them. As it is known, the diffusion ability of the dye increases as the molecular weight decreases. This may have resulted in higher penetration.
Washing and rubbing fastness tests were also performed and the results are given in Table 5. When Table 5 is examined, it is seen that the washing and rubbing fastness values of all samples are very good, except for the sample dyed with madder. Beyond the staining value, color change in washing is also a very important issue, and from this point of view, it can be said that all plants, except madder and rhubarb, give moderate to good results.
3.2 Results of Industrial Scale Dyeing
Regarding the results obtained in laboratory scale experiments, the plants giving the best results were chosen to be applied on an industrial scale as well. The selected dyes were catechu for brown, madder for red, mulberry leaf for green, and pomegranate peel for (brownish) yellow. One of the most important issues in meeting consumer expectations in terms of dyeing is the color change that will occur during repeated washings. For this reason, color changes in dyed fabrics as a result of repeated washings were tested. The results are given in Table 6.
When the color changes in repeated washings are examined, it is seen that the L* values increase slightly in repeated washings, that is, the color becomes lighter. It can be said that there is a similar tendency to decrease in b* values, that is, the nuance of the color shifts to the bluer shade. However, when evaluated in terms of natural dyeing, it can be said that the results are quite successful. To evaluate the results statistically, the changes in the L* value depending on the number of washings were evaluated with One-way ANOVA and the results of the analysis of variance are given in Table 7.
As can be seen from Table 7, washing cycle does not have statistically significant effect on the L* values, which indicates that color of the dyed fabrics does not change significantly after repeated washings. These results can be seen clearly from interval plot of L* versus washing cycle given in Fig. 3.
Washing, rubbing (dry and wet), water, light and perspiration (alkali and acid) fastness tests were also performed, and the results are given in Tables 8, 9, 10, 11.
When the fastness values are examined, it is seen that all the fastness values obtained in dyeing made with all plants are quite good, and the washing fastness of only madder (in terms of staining) is weaker than others.
When the light fastness values given in the Table 9 are examined, it is seen that the light fastnesses of catechu and mulberry leaf are quite problematic, while madder and pomegranate peel gave moderate levels of light fastness. The anthraquinone chromophore of alizarin is more stable than other chromophores to light which gave the madder a higher light fastness. About pomegranate peels, the presence of considerable tannin content in its extract increases the light fastness of the samples dyed with this colorant [44, 45].
UV protection factors have been tested to see if natural dyes contribute to providing UV protection functionality on dyed fabric samples without the need for any additional processing, and the results are given in the Table 12.
When Table 12 is examined, it is seen that the PA fabric used in dyeing actually has an excellent UV protection. It can be said that all natural dyes used in this study increased the UV protection factor of the fabric. The fabric properties such as yarn type, areal density, thickness, and weave structure affect its UV protection ability to a large extent [46, 47]. Other important factors include the type of fiber and dyes used and the finishing processes applied to the fabric. Dyes selectively absorb visible light, with most of them absorbing light between 400 and 700 nm, and some also absorbing light in the near ultraviolet range. The effect of the used dyes on UV protection of a fabric depends on the position and intensity of the UV wavelength absorption bands of the dyes and the concentration of the dyes applied on the textile substrate. The chemical structure of the dyes can affect their ability to absorb UV radiation. Polyphenolic chromophores, such as those found in certain natural dyes, have been shown to enhance the UV shielding ability of cellulosic fabrics. Also, dark colors provide better UV protection due to increased UV absorption [42, 48,49,50,51,52]. Synthetic dyes also can provide UV protection on textiles, depending on their structure and depth of shade. Improved UV protection has been obtained using disperse dyes on polyester [50], direct and reactive dyes on cotton [46, 53,54,55]. Although the fabric used in this study had UV protection properties on its own, the results showed that the natural dyes used in this study can impart UV protection to any polyamide fabric that is not UV-protective.
4 Conclusion
In this study, seven natural dye sources were applied on nylon fabric on laboratory scale and the dyeing and fastness properties were evaluated. Based on the statistical analyses, four dyes with the highest color strength and fastness properties including catechu, madder, mulberry leaf, and pomegranate peel were selected for more studies on industrial scale. The results confirmed the good color strength and satisfactory fastness properties against repeated washing, rubbing, water, and perspiration for all selected dyes. The light fastness of the samples dyed with madder and pomegranate peel were moderate while the other dyes showed very low light fastness ratings. The samples dyed with all selected natural dyes exhibited excellent protection against UV radiation. The results obtained in this study showed the potential of the selected natural dyes in eco-friendly industrial dyeing of nylon fabric with acceptable fastness properties.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
A. Haji, M. Naebe, J. Cleaner Prod. 265, 121866 (2020)
M. Klančnik, Coatings 11, 445 (2021)
K. Karabulut, R. Atav, Fibers Polym. 21, 1773 (2020)
C.T.N. Pham, H.N. Phan, T.T. Hoang, T.T.T. Dao, H.M. Bui, Res. J. Text. Apparel (2022). https://doi.org/10.1108/RJTA-07-2022-0089
R. Atav, E. Güneş, D.İ Çifçi, Y. Güneş, AATCC J. Res. 7, 15 (2020)
A. Rosyida Suranto, M. Masykuri Margono, Res. J. Text. Apparel 26, 41 (2022)
Y. Zhang, Q. Zhou, L.J. Rather, Q. Li, Ind. Crops Prod. 169, 113633 (2021)
D. Grujić, A. Savić, L. Topalić-Trivunović, M. Bizjak, A. Velemir, J. Milanović, J. Text. Inst. 114, 1206 (2023)
Y.B. Buyukakinci, E.T. Guzel, R. Karadag, Ind. Text. 72, 30 (2021)
R. Naveed, I.A. Bhatti, S. Adeel, A. Ashar, I. Sohail, M.U.H. Khan, N. Masood, M. Iqbal, A. Nazir, J. Nat. Fibers 19, 248 (2022)
A. Haji, A.N.M.A. Haque, M. Naebe, in Innovative and Emerging Technologies for Textile Dyeing and Finishing, ed. by L.J. Rather, A. Haji, M. Shabbir (Wiley-Scrivener Publishing, 2021), pp. 213
T. Gulzar, S. Adeel, I. Hanif, F. Rehman, R. Hanif, M. Zuber, N. Akhtar, J. Nat. Fibers 12, 494 (2015)
M. Verma, N. Gahlot, S.S.J. Singh, N.M. Rose, Carbohydr. Polym. 257, 117612 (2021)
L. Samant, S. Jose, N.M. Rose, D.B. Shakyawar, J. Nat. Fibers 19, 2243 (2020)
R. Atav, A.B. Soysal, F.N. Çağman, N.G. Karadağ, Color. Technol. 139, 109 (2023)
A. Haji, A. Mousavi Shoushtari, M. Mirafshar, Color. Technol. 130, 37 (2014)
F. Fan, Y. Wu, X. Wu, Color. Technol. 135, 322 (2019)
M.I. Bahtiyari, H. Benli, Ind. Text. 67, 114 (2016)
R. Atav, O. Namirti, Ind. Text. 67, 233 (2016)
A. Bouaziz, D. Dridi, S. Gargoubi, S. Chelbi, C. Boudokhane, A. Kenani, S. Aroui, Coatings 11, 1277 (2021)
R. Atav, O. Namirti, "Dyeing of Polyamide Fabrics with a Natural Dye: White Onion Skin", Fiber Society Spring 2010 International Conference, 2010
I. Ebrahimi, M. Parvinzadeh Gashti, Color. Technol. 132, 162 (2016)
M. Kanelli, M. Mandic, M. Kalakona, S. Vasilakos, D. Kekos, J. Nikodinovic-Runic, E. Topakas, Front. Microbiol. 9, 1945 (2018)
V. Jankovic, D. Markovic, J. Nikodinovic-Runic, M. Radetic, T. Ilic-Tomic, World J. Microbiol. Biotechnol. 39, 32 (2023)
M. Sadeghi-Kiakhani, S. Safapour, S. Mirnezhad, Color. Technol. 134, 308 (2018)
Y.-D. Li, J.-P. Guan, R.-C. Tang, Y.-F. Qiao, Antioxidants 8, 301 (2019)
Ö. Erdem Ismal, L. Yıldırım, Int. J. Cloth. Sci. Technol. 32, 188 (2019)
L. Yıldırım, Ö. Erdem Ìşmal, Res. J. Text. Apparel 23, 124 (2019)
K. Amutha, S.G. Annapoorani, N. Sudhapriya, Ind. Crops Prod. 148, 112303 (2020)
K. Amutha, S.G. Annapoorani, P. Sakthivel, N. Sudhapriya, J. Nat. Fibers 19, 10394 (2022)
A. Haji, F. Shahmoradi Ghaheh, L. Mohammadi, Environ. Sci. Pollut. Res. 30, 37981 (2023)
S. Lykidou, M. Pashou, E. Vouvoudi, N. Nikolaidis, Fibers Polym. 22, 3336 (2021)
M. Sadeghi-Kiakhani, A.R. Tehrani-Bagha, S. Safapour, S. Eshaghloo-Galugahi, S.M. Etezad, Environ. Dev. Sustain. 23, 9163 (2021)
F.-U. Rehman, S. Adeel, M. Pervaiz, A. Haji, W. Haddar, M. Hussaan, N. Amin, A. Guesmi, Iran. J. Chem. Chem. Eng. 40, 1849 (2021)
S. Adeel, M.U. Hasan, F. Batool, M. Ozomay, M. Hosseinnezhad, N. Amin, M. Hussaan, J. Eng. Fibers Fabr. 17, 15589250221091264 (2022)
M.U. Hasan, S. Adeel, F. Batool, T. Ahmad, R.C. Tang, N. Amin, S.R. Khan, Environ. Sci. Pollut. Res. Int. 29, 10740 (2022)
F.U. Rehman, S. Adeel, W. Haddar, R. Bibi, M. Azeem, R. Mia, B. Ahmed, Sustainability 14, 5599 (2022)
N. Eslahi, S. Maktoobi, J. Apparel Text. Sci. Technol. 11(1), 1 (2022)
A.A. Mamun, M.M. Bashar, S. Khan, M.N. Roy, M.M. Hossain, M.A. Khan, Text. Res. J. 92, 3111 (2022)
B. Ainur, B. Nurzhan, Y. Gani, N. Donyor, Ind. Text. 73, 19 (2022)
O.U. Cinko, B. Becerir, Ind. Text. 70, 248 (2019)
K. Hoffmann, J. Laperre, A. Avermaete, P. Altmeyer, T. Gambichler, Arch. Dermatol. 137, 1089 (2001)
D. Vijayalakshmi, R. Rathinamoorthy, T. Ramachandran, J. Text. Appar. Technol. Manag. 7(4), 1 (2012)
M. Hosseinnezhad, K. Gharanjig, R. Jafari, H. Imani, Prog. Color Colorants Coat. 14, 35 (2021)
A. Haji, F. Shahmoradi Ghaheh, L. Indrie, Color. Technol. 139, 165 (2023)
W.-Y. Wong, J.K.-C. Lam, C.-W. Kan, R. Postle, Text. Res. J. 86, 512 (2015)
O.K. Alebeid, T. Zhao, J. Text. Inst. 108, 2027 (2017)
M. Srinivasan, B.M. Gatewood, Text. Chem. Color. Am. Dyestuff Reporter 32(4), 36 (2000)
M.L.R. Liman, M.T. Islam, M.R. Repon, M.M. Hossain, P. Sarker, Sustainable Chem. Pharm. 21, 100417 (2021)
M. Gorenšek, F. Sluga, Text. Res. J. 74, 469 (2004)
P. Gnanavel, T. Ananthakrishnan, Man-Made Text. India 42, 127 (2014)
S. Shahidi, E. Khoshechin, S. Dalal Sharifi, R. Mongkholrattanasit, J. Nat. Fibers 19, 7213 (2022)
M. Gorenšiek, F. Sluga, R. Urbas, AATCC Rev. 7, 44 (2007)
W. Czajkowski, J. Paluszkiewicz, Fibres Text. East. Eur. 16, 122 (2008)
C.-W. Kan, C.-H. Au, Fibers Polym. 16, 1262 (2015)
Q. Meng, X. Qi, Y. Fu, Q. Chen, P. Cheng, X. Yu, X. Sun, J. Wu, W. Li, Q. Zhang, Y. Li, A. Wang, H. Bian, J. Ethnopharmacol. 248, 112326 (2020)
R. Atav, O. Namırtı, Fibers Polym. 24, 2027 (2023)
N. Nasirizadeh, H. Dehghanizadeh, M.E. Yazdanshenas, M.R. Moghadam, A. Karimi, Ind. Crops Prod. 40, 361 (2012)
Acknowledgements
The authors would like to thank TÜBİTAK for financially supporting this work within 2221 Guest or Sabbatical Scientist Support Program. The authors also would like to thank Rudolf Duraner for making the UV protection analyses.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was funded by TÜBİTAK within 2221 Guest or Sabbatical Scientist Support Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest/competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atav, R., Soysal, S. & Hajı, A. Environmentally Friendly Coloration of Polyamide Fabrics with the Use of Natural Dyes: A Study Including Results of Industrial Scale Applications. Fibers Polym 25, 2223–2232 (2024). https://doi.org/10.1007/s12221-024-00576-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-024-00576-7