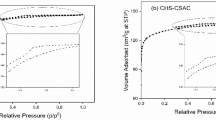
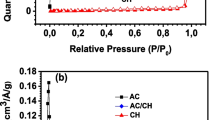
Abstract
Activated carbon (AC) obtained from peanut shell, chitosan (CH) obtained from crab shell, and prepared chitosan/ activated carbon (CH/AC) composite were studied in a batch system for the adsorption of indigo carmine (IC) from aqueous solution. Characterizations of AC, CH, and CH/AC were investigated by FTIR, SEM, XRD, zero-point charge pHpzc, thermal analysis, surface area BET, and pore-size distribution. Adsorbent weight (0.01–0.1 g), initial pH solution (2–10), initial indigo carmine concentration (10–50 mg/l) and contact time (0–60 min) were used as parameters in the adsorption equilibrium experiments. Pseudo-second-order kinetic model was found to describe the adsorption process better than pseudo-first-order kinetic model. Langmuir, Freundlich, and Temkin isotherms applied to the adsorption data reveal that AC and CH/AC best fitted Langmuir and Freundlich models when CH data fitted Temkin model with maximum adsorption capacities of 82.64 mg/g for AC, 96.15 mg/g for CH, and 208.33 mg/g for CH/AC at 30 °C. Thermodynamic parameters such as standard Gibbs free energy (ΔG°), standard enthalpy (ΔH°), and standard entropy (ΔS°) were -23.42 kJ/mol, 10.66 kJ/mol, and 112.40 J/K/mol, respectively for CH/AC. The negative value of ΔG° and a positive value of ΔH° indicate that the removal of indigo carmine by CH/AC is spontaneous and an endothermic process.
Similar content being viewed by others
References
M. Kousha, S. Tavakoli, E. Daneshvar, A. Vazirzadeh, and A. Bhatnagar, J. Mol. Liq., 207, 266 (2015).
E. Steingruber, “Indigo and Indigo Colorants”, Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, 2004.
C. F. I. Jabs, H. P. Drutz, and R. L. Summit, Am. J. Obstet. Gynecol., 185, 1368 (2001).
R. E. Palma-Goyes, J. Silva-Agredo, I. Gonzalez, and R. A. Torres-Palma, Electrochim. Acta, 140, 427 (2014).
S. Ammar, R. Abdelhedi, C. Flox, C. Arias, and E. Brillas, Environ. Chem. Lett., 4, 229 (2006).
M. M. Secula, I. Cretescu, and S. Petrescu, Desalination, 277, 227 (2011).
Y. Hu, X. Chen, Z. Liu, G. Wang, and S. Liao, J. Environ. Manage, 166, 512 (2016).
T. N. Ramesh and V. P. Sreenivasa, J. Mater., 2015, Article ID 753057 (2015). https://doi.org/10.1155/2015/753057.
S. Sanchez-Rodriguez, J. Trujillo-Reyes, E. Gutierrez-Segura, M. Solache-Rios, and A. Colin-Cruz, Sep. Sci. Technol., 50, 1602 (2015).
J. Zhang, Q. Zhou, and L. Ou, Desalin. Water Treat., 57, 718 (2016).
A. Mittal, J. Mittal, and L. Kurup, J. Hazard. Mater., 137, 591 (2006).
J. Zhang, P. Zhang, S. Zhang, and Q. Zhou, Sep. Sci. Technol., 49, 877 (2014).
F. Mbarki, A. Kesraoui, M. Seffen, and P. Ayrault, Kinetic, Water Air Soil Pollut., 229, 95 (2018).
Z. Al-Qodah and R. Shawabkah, Braz. J. Chem. Eng., 26, 127 (2009).
A. A. Aljeboree, A. N Alshirifi, and A. F. Alkaim, Arabian J. Chem., 10, S3381 (2017).
A. Khaled, A. El Nemr, A. El-Sikaily, and A. Abdelwahab, Desalination, 238, 210 (2009).
M. A. Ahmad and N. K. Rahman, Chem. Eng. J., 170, 154 (2011).
U. Gecgel and H. Kolancilar, Nat. Prod. Res., 26, 659 (2012).
Z. Z. Shahraki, H. Sharififard, and A. Lashanizadegan, Mater. Res. Express, 5, 055603 (2018).
S. Kumari, P. Rath, A. S. H. Kumar, and T. N. Tiwari, Environ. Technol. Innovation, 3, 77 (2015).
C. Muangchinda, C. Chamcheun, R. Sawatsing, and O. Pinyakong, Environ. Sci. Pollut. Res., 25, 26927 (2018).
S. Hydari, H. Sharififard, M. Nabavinia, and M. R. Parvizi, Chem. Eng. J., 193–194, 276 (2012).
S. S. Danalioglu, S. S. Bayazit, O. K. Kuyumcu, and M. A. Salam, J. Mol. Liq., 240, 589 (2017).
H. Karaer and I. Kaya, Microporous Mesoporous Mater., 232, 26 (2016).
R. Lelifajri and R. Nurfatimah, Carbohydr. Polym., 199, 499 (2018).
H. Sharififard, Z. H. Shahraki, E. Rezvanpanah, and S. R. Hosseini, Bioresour. Technol., 270, 562 (2018).
Y. Guo and D. A. Rockstraw, Microporous Mesoporous Mater., 100, 12 (2007).
H. El Knidri, R. El Khalfaouy, A. Laajeb, A. Addaou, and A. Lahsini, Process Saf. Environ. Prot., 104, 395 (2016).
H. Zhang, S. Yun, L. Song, Y. Zhang, and Y. Zhao, Int. J. Biol. Macromol., 96, 334 (2017).
J. Brugnerotto, J. Lizardi, F. M. Goycoolea, W. Arguelles-Monal, J. Desbrieres, and M. Rinaudo, Polymer, 42, 3569 (2001).
Z. Harrache, M. Abbas, T. Aksil, and M. Trari, Microchem. J., 144, 180 (2019).
S. Brunauer, P. H. Emmett, and E. Teller, J. Am. Chem. Soc., 60, 309 (1938).
G. Horvath and K. Kawazoe, J. Chem. Eng. Jpn., 16, 470 (1983).
E. E. Barrett, L.G. Joyner, and P. P. Halenda, J. Am. Chem. Soc., 73, 373 (1951).
L. Mouni, L. Belkhiri, J. C. Bollinger, A. Bouzaz, A. Assadi, A. Tirri, F. Dahmoune, K. Madani, and H. Remini, Appl. Clay Sci., 153, 38 (2018).
F. Al-Sagheer, M. Al-Sughayer, S. Muslim, and M. Z. Elsabee, Carbohydr. Polym., 77, 410 (2009).
Y. Wang, Y. Chang, L. Yu, C. Zhang, X. Xu, Y. Xue, Z. Li, and C. Xue, Carbohydr. Polym., 92, 90 (2013).
A. Kucukgulmez, M. Celik, Y. Yanar, D. Sen, H. Polat, and E. Kadak, Food Chem., 126, 1144 (2011).
M. M. Mohammed, P. Williams, and O. Tverezovskaya, Food Hydrocolloids, 31, 166 (2013).
F. Marrakchi, M. J. Ahmed, W. A. Khanday, M. Asif, and B. H. Hameed, Int. J. Biol. Macromol., 98, 233 (2017).
A. A. Ahmad, M. M. Loh, and J. A. Aziz, Dyes Pigm., 75, 263 (2007).
A. Allwar, IOSR J. Appl. Chem., 2, 9 (2012).
H. Struszczyk, J. Appl. Polym. Sci., 33, 177 (1987).
M. M. Yen, J. H. Yang, and J. L. Mau, Carbohydr. Polym., 75, 15 (2009).
K. Malins, V. Kampars, J. Brinks, I. Neibolte and R. Murnieks, I. Appl. Catal. B, 176–177, 553 (2015).
M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, R. F. Reinoso, J. Rouquerol, and K. S. W. Sing, J. P. Pure Appl. Chem., 87, 1051 (2015).
G. Crini and P. M. Badot, “Sorption Process and Pollution: Conventional and Non-conventional Sorbents”, p.497, Presses Universitaires de Franche-Comté, 2010.
D. Mitogiannis, G. Markou, A. Celekli, and H. Bozkurt, J. Environ. Chem. Eng., 3, 670 (2015).
N. A. S. Mubarak, A. H. Jawad, and W. I. Nawawi, Energy Ecol. Environ., 2, 85 (2017).
T. B. Gupta and D. H. Lataye, J. Hazard. Toxic Radioact. Waste, 21, 04017013 (2017).
T. Oymak and E. Bagda, CLEAN-Soil Air Water, 46, 1700186 (2018).
U. U. Lakshmi, V. C. Srivastava, I. D. Mall, and D. H. Lataye, J. Environ. Manage, 90, 710 (2009).
L. Zhang, Q. Liu, P. Hu, and R. Huang, Desalin. Water Treat., 57, 17011 (2016).
R. Huang, Q. Liu, J. Huo, and B. Yang, Arabian J. Chem., 10, 24 (2017).
S. Lagergren, Kungliga Svenska Vetenskapsakademiens Handlingar, 24, 1 (1898).
Y. S. Ho and G. McKay, Process Biochem., 34, 451 (1999).
W. J. Weber and J. C. Morris, J. Sanit. Eng. Div., 89, 31 (1963).
I. Langmuir, J. Am. Chem. Soc., 40, 1361 (1918).
H. Freundlich and W. Heller, J. Am. Chem. Soc., 61, 2228 (1939).
M. J. Temkin and V. Pyzhev, Acta Physicochimica URSS, 12, 217 (1940).
T. W. Weber and R. K. Chakravorti, AlChE J., 20, 228 (1974).
S. Mohan and J. Karthikeyan, Environ. Pollut., 97, 183 (1997).
S. Goldberg, “Chemical Processes in Soils”, SSSA Book Series 8, pp.489–517, Soil Science Society of America, 2005.
A. G. S. Prado, J. D. Torres, E. A. Faria, and S. C. L. Dias, J. Colloid Interface Sci., 277, 43 (2004).
J. Zolgharnein, M. Bagtash, and N. Asanjarani, J. Environ. Chem. Eng., 2, 988 (2014).
M. Bagtash and J. Zolgharnein, Wiley Chemometrics, 32, e3039 (2018).
F. S. C. dos Anjos, E. F. S. Vieira, and A. R. Cestari, J. Colloid Interface Sci., 253, 243 (2002).
M. M. Zazycki, M. Godinho, D. Perondi, E. L. Foletto, G. C. Collazzo, and G. L. Dotto, J. Cleaner Prod., 171, 57 (2018).
D. Pathania, S. Sharma, and P. Singh, Arabian J. Chem., 10, S1445 (2017).
P. Saha and S. Chowdhury in “Thermodynamics” (M. Tadashi Ed.), pp.349–364, InTech Europe, 2011.
A. Kesraoui, T. Selmi, M. Seffen, and F. Brouers, Environ. Sci. Pollut. Res., 24, 9940 (2017).
R. A. Reza and M. Ahmaruzzaman, J. Environ. Chem. Eng., 3, 395 (2015).
Acknowledgment
We gratefully acknowledge the Adminitrative authorities of “Ecole Normale Supérieure” (ENS) of Natitingou, Benin for research financing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatombi, J.K., Idohou, E.A., Osseni, S.A. et al. Adsorption of Indigo Carmine from Aqueous Solution by Chitosan and Chitosan/Activated Carbon Composite: Kinetics, Isotherms and Thermodynamics Studies. Fibers Polym 20, 1820–1832 (2019). https://doi.org/10.1007/s12221-019-1107-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-019-1107-y