Abstract
Conversion of carbon dioxide (CO2) into valuable chemicals and renewable fuels via photocatalysis represents an eco-friendly route to achieve the goal of carbon neutralization. Although various types of semiconductor materials have been intensively explored, some severe issues, such as rapid charge recombination and sluggish redox reaction kinetics, remain. In this regard, cocatalyst modification by trapping charges and boosting surface reactions is one of the most efficient strategies to improve the efficiency of semiconductor photocatalysts. This review focuses on recent advances in CO2 photoreduction over cost-effective and earth-abundant cobalt (Co)-based cocatalysts, which are competitive candidates of noble metals for practical applications. First, the functions of Co-based cocatalysts for promoting photocatalytic CO2 reduction are briefly discussed. Then, different kinds of Co-based cocatalysts, including cobalt oxides and hydroxides, cobalt nitrides and phosphides, cobalt sulfides and selenides, Co single-atom, and Co-based metal–organic frameworks (MOFs), are summarized. The underlying mechanisms of these Co-based cocatalysts for facilitating CO2 adsorption–activation, boosting charge separation, and modulating intermediate formation are discussed in detail based on experimental characterizations and density functional theory calculations. In addition, the suppression of the competing hydrogen evolution reaction using Co-based cocatalysts to promote the product selectivity of CO2 reduction is highlighted in some selected examples. Finally, the challenges and future perspectives on constructing more efficient Co-based cocatalysts for practical applications are proposed.



Reproduced with permission from Ref. [85]. Copyright © 2016 Royal Society of Chemistry. d Density functional theory (DFT) calculation of the adsorption and reduction of CO2 on Co3O4 surfaces, and free energy diagram describing the COOH* intermediate from CO2 reduction to CO on the {111} and {112} surfaces of Co3O4 hexagonal platelets. Reproduced with permission from Ref. [82]. Copyright © 2016 Wiley–VCH. e Electron paramagnetic resonance (EPR) spectra, f photocatalytic activities (λ ≥ 420 nm, 1 h), g TOF values, and h reaction mechanism of the CO2 reduction over OV–Co3O4. Reproduced with permission from Ref. [94]. Copyright © 2022 Elsevier

Reproduced with permission from Ref. [76]. Copyright © 2021 American Chemical Society. d Photocatalytic CO and O2 production rates of CdS, 0.2-Co(OH)2/CdS, 0.5-Co(OH)2/CdS, and 1-Co(OH)2/CdS within 4 h. e CO, O2, H2, and CH4 production rates of CdS and 0.5-Co(OH)2/CdS. Reproduced with permission from Ref. [97]. Copyright © 2021 Royal Society of Chemistry


Reproduced with permission from Ref. [107]. Copyright © 2021 Royal Society of Chemistry. d Illustration of the synthetic process of hierarchical FeCoS2–CoS2 DSNTs, e scanning electron microscopy image of FeCoS2–CoS2 DSNTs, f CO2 photoreduction performance of different samples, and g CO2 adsorption isotherms of FeCoS2–CoS2 DSNTs at 0 ℃. Reproduced with permission from Ref. [109]. Copyright © 2020 Wiley–VCH

Reproduced with permission from Ref. [105]. Copyright © 2021 Wiley–VCH. e Diagram of the CoP@NC synthetic process, f in situ room-temperature PL spectra, and g time-resolved PL spectra of the Ru solution in the absence/presence of CoP or CoP@NC. Reproduced with permission from Ref. [106]. Copyright © 2021 Wiley–VCH. h Relative energy change diagram for the CO2 reduction to CO catalyzed by CoP in the system containing a proton donor and i yields of CO and H2. Reproduced with permission from Ref. [103]. Copyright © 2018 Wiley–VCH

Reproduced with permission from Ref. [117]. Copyright © 2021 Elsevier. d PL spectra of [Ru(bpy)3]Cl2·6H2O and [Ru(bpy)3]Cl2·6H2O + Co-SA@SP-800, e CO and H2 yields of the M-SA@SP-800 (M = Fe, Ni, and Cu), and f CO and H2 yield rates from the CO2 photoreduction under various reaction conditions. Reproduced with permission from Ref. [116]. Copyright © 2021 Royal Society of Chemistry

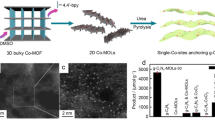
Reproduced with permission from Ref. [208]. Copyright © 2021 Springer. b Chemical structure of Co-ZIF-9. Ball-and-stick representation of the second building units showing the coordination environment around cobalt (left) and packing diagram of Co-ZIF-9 (right). Reproduced with permission from Ref. [123]. Copyright © 2014 Wiley–VCH

Reproduced with permission from Ref. [130]. Copyright © 2018 Royal Society of Chemistry. f Comparison of the photocurrent–potential curves of different samples. Reproduced with permission from Ref. [126]. Copyright © 2016 Royal Society of Chemistry. g Photocatalytic production of CO and H2 catalyzed by MOF-Cu, MOF-Co, and MOF-Ni. Reproduced with permission from Ref. [132]. Copyright © 2019 American Chemical Society
Similar content being viewed by others
References
Cao JH, Law SH, Wu DS et al (2022) Does environmental regulation promote the volatility of technological progress?—Analysis based on the law of entropy generation. Front Environ Sci 10:876707
Mazzone V, Bonifazi M, Aegerter CM et al (2021) Clean carbon cycle via high-performing and low-cost solar-driven production of freshwater. Adv Sustain Syst 5(10):202100217
Mansouri NY, Alhusseini A, Al-Saud NT et al (2020) A carbon management system of innovation: towards a circular carbon economy. In: G20 virtual summit, Riyadh, Saudi Arabia https://www.g20-insights.org/wp-content/uploads/2020/12/a-carbon-management-system-of-innovation-towards-a-circular-carbon-economy-1607598005.pdf
Yang Y, Zhang Y, Hu JS et al (2020) Progress in the mechanisms and materials for CO2 electroreduction toward C2+ products. Acta Phys-Chim Sin 36(1):1906085–1906080
Wang N, Miao RK, Lee G et al (2021) Suppressing the liquid product crossover in electrochemical CO2 reduction. SmartMat 2(1):12–16
Chen TR, Weng B, Lu SW et al (2022) Photocatalytic anaerobic dehydrogenation of alcohols over metal halide perovskites: a new acid-free scheme for H2 production. J Phys Chem Lett 13(28):6559–6565
Lu SW, Weng B, Chen AZ et al (2021) Facet engineering of Pd nanocrystals for enhancing photocatalytic hydrogenation: modulation of the Schottky barrier height and enrichment of surface reactants. ACS Appl Mater Interfaces 13(11):13044–13054
Huang ZQ, Wang JQ, Lu SW et al (2021) Insight into the real efficacy of graphene for enhancing photocatalytic efficiency: a case study on CVD graphene-TiO2 composites. ACS Appl Energy Mater 4(9):8755–8764
Simakov DSA (2017) Renewable synthetic fuels and chemicals from carbon dioxide. Springer, Cham, p 69
Yuan L, Xu YJ (2015) Photocatalytic conversion of CO2 into value-added and renewable fuels. Appl Surf Sci 342:154–167
Lu YB, Zhang ZH, Wang HM et al (2021) Toward efficient single-atom catalysts for renewable fuels and chemicals production from biomass and CO2. Appl Catal B Environ 292:120162
Albero J, Peng Y, García H (2020) Photocatalytic CO2 reduction to C2+ products. ACS Catal 10(10):5734–5749
Li K, Peng BS, Peng TY (2016) Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal 6(11):7485–7527
Kreft S, Wei D, Junge H et al (2020) Recent advances on TiO2-based photocatalytic CO2 reduction. EnergyChem 2(6):100044
Shi R, Waterhouse GIN, Zhang TR (2017) Recent progress in photocatalytic CO2 reduction over perovskite oxides. Sol RRL 1(11):1700126
Zhang YZ, Xia BQ, Ran JR et al (2020) Atomic-level reactive sites for semiconductor-based photocatalytic CO2 reduction. Adv Energy Mater 10(9):1903879
Sun ZX, Wang HQ, Wu ZB et al (2018) g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal Today 300:160–172
Li R, Zhang W, Zhou K (2018) Metal-organic-framework-based catalysts for photoreduction of CO2. Adv Mater 30(35):e1705512
Marszewski M, Cao SW, Yu JG et al (2015) Semiconductor-based photocatalytic CO2 conversion. Mater Horiz 2(3):261–278
Zhang TX, Wang T, Meng FL et al (2022) Recent advances in ZnIn2S4-based materials towards photocatalytic purification, solar fuel production and organic transformations. J Mater Chem C 10(14):5400–5424
Wang Q, Pan ZH (2022) Advances and challenges in developing cocatalysts for photocatalytic conversion of carbon dioxide to fuels. Nano Res 1–20. https://doi.org/10.1007/s12274-022-4705-8
Ong WJ, Putri LK, Mohamed AR (2020) Rational design of carbon-based 2D nanostructures for enhanced photocatalytic CO2 reduction: a dimensionality perspective. Chemistry 26(44):9710–9748
Wang T, Meng XG, Liu GG et al (2015) In situ synthesis of ordered mesoporous Co-doped TiO2 and its enhanced photocatalytic activity and selectivity for the reduction of CO2. J Mater Chem A 3(18):9491–9501
Beigi AA, Fatemi S, Salehi Z (2014) Synthesis of nanocomposite CdS/TiO2 and investigation of its photocatalytic activity for CO2 reduction to CO and CH4 under visible light irradiation. J CO2 Util 7:23–29
Yan YB, Yu YL, Wu D et al (2016) TiO2/vanadate (Sr10V6O25, Ni3V2O8, Zn2V2O7) heterostructured photocatalysts with enhanced photocatalytic activity for photoreduction of CO2 into CH4. Nanoscale 8(2):949–958
Ji YF, Luo Y (2016) New mechanism for photocatalytic reduction of CO2 on the anatase TiO2 (101) surface: the essential role of oxygen vacancy. J Am Chem Soc 138(49):15896–15902
Wang WN, An WJ, Ramalingam B et al (2012) Size and structure matter: enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J Am Chem Soc 134(27):11276–11281
Li X, Yu JG, Jaroniec M et al (2019) Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem Rev 119(6):3962–4179
Chang XX, Wang T, Yang PP et al (2019) The development of cocatalysts for photoelectrochemical CO2 reduction. Adv Mater 31(31):e1804710
Ran J, Jaroniec M, Qiao SZ (2018) Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities. Adv Mater 30(7):1704649
Cao SW, Li Y, Zhu BC et al (2017) Facet effect of Pd cocatalyst on photocatalytic CO2 reduction over g-C3N4. J Catal 349:208–217
Zhu YZ, Gao C, Bai S et al (2017) Hydriding Pd cocatalysts: an approach to giant enhancement on photocatalytic CO2 reduction into CH4. Nano Res 10(10):3396–3406
Wu HK, Li YH, Qi MY et al (2020) Enhanced photocatalytic CO2 reduction with suppressing H2 evolution via Pt cocatalyst and surface SiO2 coating. Appl Catal B Environ 278:119267
Chen YX, Xu YF, Wang XD et al (2020) Solvent selection and Pt decoration towards enhanced photocatalytic CO2 reduction over CsPbBr3 perovskite single crystals. Sustain Energy Fuels 4(5):2249–2255
Guo K, Zhu XL, Peng LL et al (2021) Boosting photocatalytic CO2 reduction over a covalent organic framework decorated with ruthenium nanoparticles. Chem Eng J 405:127011
Baran T, Wojtyła S, Dibenedetto A et al (2015) Zinc sulfide functionalized with ruthenium nanoparticles for photocatalytic reduction of CO2. Appl Catal B Environ 178:170–176
Chen S, Pan B, Zeng LQ et al (2017) La2Sn2O7 enhanced photocatalytic CO2 reduction with H2O by deposition of Au co-catalyst. RSC Adv 7(23):14186–14191
Wang R, Shen J, Sun KH et al (2019) Enhancement in photocatalytic activity of CO2 reduction to CH4 by 0D/2D Au/TiO2 plasmon heterojunction. Appl Surf Sci 493:1142–1149
You JK, Xiao M, Wang ZL et al (2022) Non-noble metal-based cocatalysts for photocatalytic CO2 reduction. J CO2 Util 55:101817
Wu SQ, Wang JB, Li QC et al (2021) Bi/BiOCl nanosheets enriched with oxygen vacancies to enhance photocatalytic CO2 reduction. Trans Tianjin Univ 27(2):155–164
Liu C, Cundari TR, Wilson AK (2012) CO2 reduction on transition metal (Fe Co, Ni, and Cu) surfaces: in comparison with homogeneous catalysis. J Phys Chem C 116(9):5681–5688
Zhao H, Jian L, Gong M et al (2022) Transition-metal-based cocatalysts for photocatalytic water splitting. Small Struct 3(7):2100229
Huang SL, Yi H, Zhang LH et al (2020) Non-precious molybdenum nanospheres as a novel cocatalyst for full-spectrum-driven photocatalytic CO2 reforming to CH4. J Hazard Mater 393:122324
Xu YF, Duchesne PN, Wang L et al (2020) High-performance light-driven heterogeneous CO2 catalysis with near-unity selectivity on metal phosphides. Nat Commun 11(1):5149
Zhang XD, Yan J, Zheng FY et al (2021) Designing charge transfer route at the interface between WP nanoparticle and g-C3N4 for highly enhanced photocatalytic CO2 reduction reaction. Appl Catal B Environ 286:119879
Li XW, Lu SW, Yi JY et al (2022) Ultrathin two-dimensional ZnIn2S4/Nix-B heterostructure for high-performance photocatalytic fine chemical synthesis and H2 generation. ACS Appl Mater Interfaces 14(22):25297–25307
Li BF, Wei F, Su B et al (2022) Mesoporous cobalt tungstate nanoparticles for efficient and stable visible-light-driven photocatalytic CO2 reduction. Mater Today Energy 24:100943
Li XW, Li MQ, Liu JG et al (2022) Amorphous nickel borate as a high-efficiency cocatalyst for H2 generation and fine chemical synthesis. Catal Commun 162:106389
Yang G, Guan SY, Mehdi S et al (2021) Co-CoOx supported onto TiO2 coated with carbon as a catalyst for efficient and stable hydrogen generation from ammonia borane. Green Energy Environ 6(2):236–243
Wu Y, Guo YR, Yu HG et al (2021) Nonstoichiometric yttrium hydride–promoted reversible hydrogen storage in a liquid organic hydrogen carrier. CCS Chem 3(3):974–984
Ma MZ, Chen JH, Huang ZA et al (2022) Intermolecular hydrogen bond modulating the selective coupling of protons and CO2 to CH4 over nitrogen-doped carbon layers modified cobalt. Chem Eng J 444:136585
Padamata SK, Yasinskiy AS, Polyakov PV et al (2020) Recovery of noble metals from spent catalysts: a review. Metall Mater Trans B 51(5):2413–2435
Pourret O, Faucon MP (2018) Encyclopedia of geochemistry. Springer, Cham, p 1557
Saunders JE, Pearson NJ, O’Reilly SY et al (2018) Gold in the mantle: a global assessment of abundance and redistribution processes. Lithos 322:376–391
Singh AK, Mukherjee R (2008) Cobalt(ii) and cobalt(iii) complexes of thioether-containing hexadentate pyrazine amide ligands: C–S bond cleavage and cyclometallation reaction. Dalton Trans 2:260–270
Usman M, Humayun M, Garba MD et al (2021) Electrochemical reduction of CO2: a review of cobalt based catalysts for carbon dioxide conversion to fuels. Nanomaterials (Basel) 11(8):2029
Nguyen DT, Nguyen CC, Do TO (2020) Rational one-step synthesis of cobalt clusters embedded-graphitic carbon nitrides for the efficient photocatalytic CO2 reduction under ambient conditions. J Catal 392:88–96
Wannakao S, Jumpathong W, Kongpatpanich K (2017) Tailoring metalloporphyrin frameworks for an efficient carbon dioxide electroreduction: selectively stabilizing key intermediates with H-bonding pockets. Inorg Chem 56(12):7200–7209
Mao J, Li K, Peng TY (2013) Recent advances in the photocatalytic CO2 reduction over semiconductors. Catal Sci Technol 3(10):2481
Sun MY, Zhao BH, Chen FP et al (2021) Thermally-assisted photocatalytic CO2 reduction to fuels. Chem Eng J 408:127280
Kuehnel MF, Orchard KL, Dalle KE et al (2017) Selective photocatalytic CO2 reduction in water through anchoring of a molecular Ni catalyst on CdS nanocrystals. J Am Chem Soc 139(21):7217–7223
Markovits A, Fahmi A, Minot C (1996) A theoretical study of CO2 adsorption on TiO2. J Mol Struct Theochem 371:219–235
Su PP, Iwase K, Harada T et al (2018) Covalent triazine framework modified with coordinatively-unsaturated Co or Ni atoms for CO2 electrochemical reduction. Chem Sci 9(16):3941–3947
Yu HY, Cao DP, Fisher A et al (2017) Size effect on the adsorption and dissociation of CO2 on Co nanoclusters. Appl Surf Sci 396:539–546
Chen P, Zhang YX, Zhou Y et al (2021) Photoelectrocatalytic carbon dioxide reduction: fundamental, advances and challenges. Nano Mater Sci 3(4):344–367
Wang JJ, Lin S, Tian N et al (2021) Nanostructured metal sulfides: classification, modification strategy, and solar-driven CO2 reduction application. Adv Funct Mater 31(9):2008008
Li J, Zhang LZ, Li YJ et al (2014) Synthesis and internal electric field dependent photoreactivity of Bi3O4Cl single-crystalline nanosheets with high 001 facet exposure percentages. Nanoscale 6(1):167–171
Zhan WW, Gao H, Yang Y et al (2022) Rational design of metal-organic framework-based materials for photocatalytic CO2 reduction. Adv Energy Sustain Res 3(7):2200004
Li JG, Wan WC, Triana CA et al (2019) Dynamic role of cluster cocatalysts on molecular photoanodes for water oxidation. J Am Chem Soc 141(32):12839–12848
Wang ZZ, Rong JY, Lv JQ et al (2021) Chelation-mediated in situ formation of ultrathin cobalt (oxy)hydroxides on hematite photoanode towards enhanced photoelectrochemical water oxidation. J Energy Chem 56:152–161
Fu JW, Jiang KX, Qiu XQ et al (2020) Product selectivity of photocatalytic CO2 reduction reactions. Mater Today 32:222–243
Goren Z, Willner I, Nelson AJ et al (1990) Selective photoreduction of carbon dioxide/bicarbonate to formate by aqueous suspensions and colloids of palladium-titania. J Phys Chem 94(9):3784–3790
Willner I, Maidan RB, Mandler D et al (1987) Photosensitized reduction of carbon dioxide to methane and hydrogen evolution in the presence of ruthenium and osmium colloids: strategies to design selectivity of products distribution. J Am Chem Soc 109(20):6080–6086
Di J, Chen C, Zhu C et al (2021) Cobalt nitride as a novel cocatalyst to boost photocatalytic CO2 reduction. Nano Energy 79:105429
Di J, Chen C, Yang SZ et al (2019) Isolated single atom cobalt in Bi3O4Br atomic layers to trigger efficient CO2 photoreduction. Nat Commun 10(1):2840
Liao WR, Chen WH, Lu SW et al (2021) Alkaline Co(OH)2-decorated 2D monolayer titanic acid nanosheets for enhanced photocatalytic syngas production from CO2. ACS Appl Mater Interfaces 13(32):38239–38247
Lee DS, Chen HJ, Chen YW (2012) Photocatalytic reduction of carbon dioxide with water using InNbO4 catalyst with NiO and Co3O4 cocatalysts. J Phys Chem Solids 73(5):661–669
Lin JL, Pan ZM, Wang XC (2014) Photochemical reduction of CO2 by graphitic carbon nitride polymers. ACS Sustain Chem Eng 2(3):353–358
Wang SB, Ding ZX, Wang XC (2015) A stable ZnCo2O4 cocatalyst for photocatalytic CO2 reduction. Chem Commun (Camb) 51(8):1517–1519
Wang SB, Hou YD, Wang XC (2015) Development of a stable MnCo2O4 cocatalyst for photocatalytic CO2 reduction with visible light. ACS Appl Mater Interfaces 7(7):4327–4335
Jiang M, Gao YL, Wang ZY et al (2016) Photocatalytic CO2 reduction promoted by a CuCo2O4 cocatalyst with homogeneous and heterogeneous light harvesters. Appl Catal B Environ 198:180–188
Gao C, Meng QQ, Zhao K et al (2016) Co3O4 hexagonal platelets with controllable facets enabling highly efficient visible-light photocatalytic reduction of CO2. Adv Mater 28(30):6485–6490
Dong CY, Xing MY, Zhang JL (2016) Double-cocatalysts promote charge separation efficiency in CO2 photoreduction: spatial location matters. Mater Horiz 3(6):608–612
Lin F, Gao MC, Wang YL et al (2022) Enhanced mass transfer with amphiphilic CoOx/biochar for photocatalytic CO2 conversion. Appl Surf Sci 605:154612
Wang T, Shi L, Tang J et al (2016) A Co3O4-embedded porous ZnO rhombic dodecahedron prepared using zeolitic imidazolate frameworks as precursors for CO2 photoreduction. Nanoscale 8(12):6712–6720
Chen WY, Han B, Tian C et al (2019) MOFs-derived ultrathin holey Co3O4 nanosheets for enhanced visible light CO2 reduction. Appl Catal B Environ 244:996–1003
Qin JN, Lin LH, Wang XC (2018) A perovskite oxide LaCoO3 cocatalyst for efficient photocatalytic reduction of CO2 with visible light. Chem Commun (Camb) 54(18):2272–2275
Zhu SY, Liao WR, Zhang MY et al (2019) Design of spatially separated Au and CoO dual cocatalysts on hollow TiO2 for enhanced photocatalytic activity towards the reduction of CO2 to CH4. Chem Eng J 361:461–469
Ma YW, Du J, Fang YX et al (2021) Encapsulation of cobalt oxide into metal-organic frameworks for an improved photocatalytic CO2 reduction. Chemsuschem 14(3):946–951
Sun WJ, Meng XY, Xu CJ et al (2020) Amorphous CoOx coupled carbon dots as a spongy porous bifunctional catalyst for efficient photocatalytic water oxidation and CO2 reduction. Chin J Catal 41(12):1826–1836
Garay-Rodríguez LF, Torres-Martínez LM (2020) Extending the visible-light photocatalytic CO2 reduction activity of K2Ti6O13 with the MxOy (M = Co, Ni and Cu) incorporation. J Mater Sci Mater Electron 31(21):19248–19265
Ren JT, Zheng YL, Yuan K et al (2020) Self-templated synthesis of Co3O4 hierarchical nanosheets from a metal-organic framework for efficient visible-light photocatalytic CO2 reduction. Nanoscale 12(2):755–762
He L, Zhang WY, Liu S et al (2021) Three-dimensional porous N-doped graphitic carbon framework with embedded CoO for photocatalytic CO2 reduction. Appl Catal B Environ 298:120546
Zhang Q, Yang PJ, Zhang HX et al (2022) Oxygen vacancies in Co3O4 promote CO2 photoreduction. Appl Catal B Environ 300:120729
Huang Y, Li K, Zhou JC et al (2022) Nitrogen-stabilized oxygen vacancies in TiO2 for site-selective loading of Pt and CoOx cocatalysts toward enhanced photoreduction of CO2 to CH4. Chem Eng J 439:135744
Zheng YL, Zhou L, Sun XC et al (2022) Visible-light driven CO2 reduction to CO by Co3O4 supported on tungsten oxide. J Phys Chem C 126(6):3017–3028
Xu YY, Xie ZK, Yu R et al (2021) Co(OH)2 water oxidation cocatalyst-decorated CdS nanowires for enhanced photocatalytic CO2 reduction performance. Dalton Trans 50(29):10159–10167
Lin XH, Gao YL, Jiang M et al (2018) Photocatalytic CO2 reduction promoted by uniform perovskite hydroxide CoSn(OH)6 nanocubes. Appl Catal B Environ 224:1009–1016
Lu KQ, Li YH, Zhang F et al (2020) Rationally designed transition metal hydroxide nanosheet arrays on graphene for artificial CO2 reduction. Nat Commun 11(1):5181
Chen WY, Han B, Xie YL et al (2020) Ultrathin Co-Co LDHs nanosheets assembled vertically on MXene: 3D nanoarrays for boosted visible-light-driven CO2 reduction. Chem Eng J 391:123519
Liu HH, Zhang F, Wang HF et al (2021) Oxygen vacancy engineered unsaturated coordination in cobalt carbonate hydroxide nanowires enables highly selective photocatalytic CO2 reduction. Energy Environ Sci 14(10):5339–5346
Yang PJ, Wang RR, Tao HL et al (2021) Cobalt nitride anchored on nitrogen-rich carbons for efficient carbon dioxide reduction with visible light. Appl Catal B Environ 280:119454
Fu ZC, Xu RC, Moore JT et al (2018) Highly efficient photocatalytic system constructed from CoP/carbon nanotubes or graphene for visible-light-driven CO2 reduction. Chemistry 24(17):4273–4278
Wang Y, Wang SB, Lou XWD (2019) Dispersed nickel cobalt oxyphosphide nanoparticles confined in multichannel hollow carbon fibers for photocatalytic CO2 reduction. Angew Chem Int Ed Engl 58(48):17236–17240
Niu PP, Pan ZM, Wang SB et al (2021) Tuning crystallinity and surface hydrophobicity of a cobalt phosphide cocatalyst to boost CO2 photoreduction performance. Chemsuschem 14(5):1302–1307
Niu PP, Pan ZM, Wang SB et al (2021) Cobalt phosphide cocatalysts coated with porous N-doped carbon layers for photocatalytic CO2 reduction. ChemCatChem 13(16):3581–3587
Lin XH, Xie ZD, Su B et al (2021) Well-defined Co9S8 cages enable the separation of photoexcited charges to promote visible-light CO2 reduction. Nanoscale 13(43):18070–18076
Xu Y, Long JF, Tu LX et al (2021) CoO engineered Co9S8 catalyst for CO2 photoreduction with accelerated electron transfer endowed by the built-in electric field. Chem Eng J 426:131849
Wang Y, Wang SB, Zhang SL et al (2020) Formation of hierarchical FeCoS2-CoS2 double-shelled nanotubes with enhanced performance for photocatalytic reduction of CO2. Angew Chem Int Ed Engl 59(29):11918–11922
Pan B, Wu Y, Qin JN et al (2019) Ultrathin Co0.85Se nanosheet cocatalyst for visible-light CO2 photoreduction. Catal Today 335:208–213
Xu Y, Mo J, Xie GQ et al (2020) The main factor to improve the performance of CoSe2 for photocatalytic CO2 reduction: element doping or phase transformation. J Mater Chem A 8(8):4457–4463
Zhang HB, Wei J, Dong JC et al (2016) Efficient visible-light-driven carbon dioxide reduction by a single-atom implanted metal-organic framework. Angew Chem Int Ed Engl 55(46):14310–14314
Gao C, Chen SM, Wang Y et al (2018) Heterogeneous single-atom catalyst for visible-light-driven high-turnover CO2 reduction: the role of electron transfer. Adv Mater 30(13):e1704624
Shi L, Ren XH, Wang Q et al (2020) Stabilizing atomically dispersed catalytic sites on tellurium nanosheets with strong metal-support interaction boosts photocatalysis. Small 16(35):e2002356
Fu JW, Zhu L, Jiang KX et al (2021) Activation of CO2 on graphitic carbon nitride supported single-atom cobalt sites. Chem Eng J 415:128982
Zhang PP, Zhan XN, Xu LB et al (2021) Mass production of a single-atom cobalt photocatalyst for high-performance visible-light photocatalytic CO2 reduction. J Mater Chem A 9(46):26286–26297
Chen YH, Qi MY, Li YH et al (2021) Activating two-dimensional Ti3C2Tx-MXene with single-atom cobalt for efficient CO2 photoreduction. Cell Rep Phys Sci 2(3):100371
Zhang HB, Wang Y, Zuo SW et al (2021) Isolated cobalt centers on W18O49 nanowires perform as a reaction switch for efficient CO2 photoreduction. J Am Chem Soc 143(5):2173–2177
Ren XH, Shi L, Li YX et al (2020) Single cobalt atom anchored black phosphorous nanosheets as an effective cocatalyst promotes photocatalysis. ChemCatChem 12(15):3870–3879
Ma MZ, Huang ZA, Doronkin DE et al (2022) Ultrahigh surface density of Co-N2C single-atom-sites for boosting photocatalytic CO2 reduction to methanol. Appl Catal B Environ 300:120695
Huang PP, Huang JH, Li JY et al (2022) Revealing the structure of single cobalt sites in carbon nitride for photocatalytic CO2 reduction. J Phys Chem C 126(20):8596–8604
Cheng L, Yue XY, Fan JJ et al (2022) Site-specific electron-driving observations of CO2-to-CH4 photoreduction on co-doped CeO2/crystalline carbon nitride S-scheme heterojunctions. Adv Mater 34(27):e2200929
Wang SB, Yao WS, Lin JL et al (2014) Cobalt imidazolate metal-organic frameworks photosplit CO2 under mild reaction conditions. Angew Chem Int Ed Engl 53(4):1034–1038
Wang SB, Lin JL, Wang XC (2014) Semiconductor-redox catalysis promoted by metal-organic frameworks for CO2 reduction. Phys Chem Chem Phys 16(28):14656–14660
Wang SB, Wang XC (2015) Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl Catal B Environ 162:494–500
Yan SS, Ouyang SX, Xu H et al (2016) Co-ZIF-9/TiO2 nanostructure for superior CO2 photoreduction activity. J Mater Chem A 4(39):15126–15133
Qin JN, Wang SB, Wang XC (2017) Visible-light reduction CO2 with dodecahedral zeolitic imidazolate framework ZIF-67 as an efficient co-catalyst. Appl Catal B Environ 209:476–482
Wang Y, Huang NY, Shen JQ et al (2018) Hydroxide ligands cooperate with catalytic centers in metal-organic frameworks for efficient photocatalytic CO2 reduction. J Am Chem Soc 140(1):38–41
Kong ZC, Liao JF, Dong YJ et al (2018) Core@Shell CsPbBr3@Zeolitic imidazolate framework nanocomposite for efficient photocatalytic CO2 reduction. ACS Energy Lett 3(11):2656–2662
Wang M, Liu JX, Guo CM et al (2018) Metal–organic frameworks (ZIF-67) as efficient cocatalysts for photocatalytic reduction of CO2: the role of the morphology effect. J Mater Chem A 6(11):4768–4775
Yuan XZ, Mu QQ, Xue SL et al (2021) Polypyrrole reinforced ZIF-67 with modulated facet exposure and billion-fold electrical conductivity enhancement towards robust photocatalytic CO2 reduction. J Energy Chem 60:202–208
Wang XK, Liu J, Zhang L et al (2019) Monometallic catalytic models hosted in stable metal–organic frameworks for tunable CO2 photoreduction. ACS Catal 9(3):1726–1732
Zhang JH, Wang YC, Wang HJ et al (2022) Enhancing photocatalytic performance of metal-organic frameworks for CO2 reduction by a bimetallic strategy. Chin Chem Lett 33(4):2065–2068
Sung PH, Huang CY, Lin CY et al (2022) Photocatalytic CO2 reduction for C2–C3 oxy-compounds on ZIF-67 derived carbon with TiO2. J CO2 Util 58:101920
Mangrulkar PA, Joshi MM, Tijare SN et al (2012) Nano cobalt oxides for photocatalytic hydrogen production. Int J Hydrog Energy 37(13):10462–10466
Efremova A, Szenti I, Kiss J et al (2022) Nature of the Pt-cobalt-oxide surface interaction and its role in the CO2 methanation. Appl Surf Sci 571:151326
Choi JY, Lim CK, Park B et al (2019) Surface activation of cobalt oxide nanoparticles for photocatalytic carbon dioxide reduction to methane. J Mater Chem A 7(25):15068–15072
Feng ZY, Zhu XW, Yang JM et al (2022) Inherent facet-dominant effect for cobalt oxide nanosheets to enhance photocatalytic CO2 reduction. Appl Surf Sci 578:151848
Mei J, Liao T, Ayoko GA et al (2019) Cobalt oxide-based nanoarchitectures for electrochemical energy applications. Prog Mater Sci 103:596–677
Chu JY, Sun GJ, Han XJ et al (2019) Ultrafine CoO nanoparticles as an efficient cocatalyst for enhanced photocatalytic hydrogen evolution. Nanoscale 11(33):15633–15640
Hagelin-Weaver HAE, Hoflund GB, Minahan DM et al (2004) Electron energy loss spectroscopic investigation of Co metal, CoO, and Co3O4 before and after Ar+ bombardment. Appl Surf Sci 235(4):420–448
Singh V, Major DT (2016) Electronic structure and bonding in Co-based single and mixed valence oxides: a quantum chemical perspective. Inorg Chem 55(7):3307–3315
Qu JF, Li SQ, Yang XG et al (2022) Hollow porous Co–Ni spinel nanosheet arrays with rich oxygen defects on carbon cloth toward highly efficient and selective CO2 photofixation. Carbon 200:149–155
Lei B, Cui W, Chen P et al (2022) C–doping induced oxygen-vacancy in WO3 nanosheets for CO2 activation and photoreduction. ACS Catal 12(15):9670–9678
Wang M, Shen M, Jin XX et al (2019) Oxygen vacancy generation and stabilization in CeO2–x by Cu introduction with improved CO2 photocatalytic reduction activity. ACS Catal 9(5):4573–4581
Xu H, Li SS, Ge L et al (2017) In-situ synthesis of novel plate-like Co(OH)2 co-catalyst decorated TiO2 nanosheets with efficient photocatalytic H2 evolution activity. Int J Hydrog Energy 42(36):22877–22886
Wender H, Gonçalves RV, Dias CS et al (2013) Photocatalytic hydrogen production of Co(OH)2 nanoparticle-coated α-Fe2O3 nanorings. Nanoscale 5(19):9310–9316
Sahoo DP, Nayak S, Reddy KH et al (2018) Fabrication of a Co(OH)2/ZnCr LDH “p-n” heterojunction photocatalyst with enhanced separation of charge carriers for efficient visible-light-driven H2 and O2 evolution. Inorg Chem 57(7):3840–3854
Zhang GG, Zang SH, Wang XC (2015) Layered Co(OH)2 deposited polymeric carbon nitrides for photocatalytic water oxidation. ACS Catal 5(2):941–947
Kim SJ, Lee Y, Lee DK et al (2014) Efficient Co–Fe layered double hydroxide photocatalysts for water oxidation under visible light. J Mater Chem A 2(12):4136
Cui HT, Zhao YN, Ren WZ et al (2013) Large scale selective synthesis of α-Co(OH)2 and β-Co(OH)2 nanosheets through a fluoride ions mediated phase transformation process. J Alloys Compd 562:33–37
Li JB, Li ZH, Zhan F et al (2020) Phase engineering of cobalt hydroxide toward cation intercalation. Chem Sci 12(5):1756–1761
Cheng JP, Liu L, Zhang J et al (2014) Influences of anion exchange and phase transformation on the supercapacitive properties of α-Co(OH)2. J Electroanal Chem 722–723:23–31
Wang MM, Chen DY, Li NJ et al (2022) Ni-Co bimetallic hydroxide nanosheet arrays anchored on graphene for adsorption-induced enhanced photocatalytic CO2 reduction. Adv Mater 34(28):e2202960
Sibokoza SB, Moloto MJ, Moloto N (2011) Synthesis and characterization of reproducible stoichiometry of cobalt sulfide nanoparticles using sulphur containing single-source precursors. In: The 56th annual conference of the SA Institute of Physics, Johannesburg, Africa https://www.researchgate.net/publication/281377947
Kristl M, Dojer B, Gyergyek S et al (2017) Synthesis of nickel and cobalt sulfide nanoparticles using a low cost sonochemical method. Heliyon 3(3):e00273
Alnaggar GAN, Ananda S (2019) Electrochemical synthesis of 3D hierarchical Co3S4/Co9S8 nanoparticles as photocatalysts for degradation of Carboxylic acids. Int J Appl Chem 15(2):81–97
Manjunatha C, Lakshmikant S, Shreenivasa L et al (2021) Development of non-stoichiometric hybrid Co3S4/Co0.85Se nanocomposites for an evaluation of synergistic effect on the OER performance. Surf Interfaces 25:101161
Hashmi S, Singh M, Weerathunge P et al (2021) Cobalt sulfide nanosheets as peroxidase mimics for colorimetric detection of l-cysteine. ACS Appl Nano Mater 4(12):13352–13362
Bao SJ, Li YB, Li CM et al (2008) Shape evolution and magnetic properties of cobalt sulfide. Cryst Growth Des 8(10):3745–3749
Liu X, Zhang K, Lei KX et al (2016) Facile synthesis and electrochemical sodium storage of CoS2 micro/nano-structures. Nano Res 9(1):198–206
Wang ZP, Lin ZP, Deng J et al (2021) Elevating the d-band center of six-coordinated octahedrons in Co9S8 through Fe-incorporated topochemical deintercalation. Adv Energy Mater 11(5):2003023
Bouwens SMAM, Van Veen JAR, Koningsberger DC et al (1991) EXAFS determination of the structure of cobalt in carbon-supported cobalt and cobalt-molybdenum sulfide hydrodesulfurization catalysts. J Phys Chem 95(1):123–134
Tajik S, Dourandish Z, Garkani Nejad F et al (2022) Transition metal dichalcogenides: synthesis and use in the development of electrochemical sensors and biosensors. Biosens Bioelectron 216:114674
Han F, Zhang CZ, Sun B et al (2017) Dual-carbon phase-protective cobalt sulfide nanoparticles with cable-type and mesoporous nanostructure for enhanced cycling stability in sodium and lithium ion batteries. Carbon 118:731–742
Jiao XC, Li XD, Jin XY et al (2017) Partially oxidized SnS2 atomic layers achieving efficient visible-light-driven CO2 reduction. J Am Chem Soc 139(49):18044–18051
Peng X, Yan YJ, Jin X et al (2020) Recent advance and prospectives of electrocatalysts based on transition metal selenides for efficient water splitting. Nano Energy 78:105234
Xia XY, Wang LJ, Sui N et al (2020) Recent progress in transition metal selenide electrocatalysts for water splitting. Nanoscale 12(23):12249–12262
Wan S, Jin WY, Guo XL et al (2018) Self-templating construction of porous CoSe2 nanosheet arrays as efficient bifunctional electrocatalysts for overall water splitting. ACS Sustain Chem Eng 6(11):15374–15382
Nithya VD (2021) Recent advances in CoSe2 electrocatalysts for hydrogen evolution reaction. Int J Hydrog Energy 46(73):36080–36102
Li ML, Shu CZ, Hu AJ et al (2020) Invigorating the catalytic activity of cobalt selenide via structural phase transition engineering for lithium–oxygen batteries. ACS Sustain Chem Eng 8(13):5018–5027
Kong DS, Wang HT, Lu ZY et al (2014) CoSe2 nanoparticles grown on carbon fiber paper: an efficient and stable electrocatalyst for hydrogen evolution reaction. J Am Chem Soc 136(13):4897–4900
Zhang GG, Zang SH, Lan ZA et al (2015) Cobalt selenide: a versatile cocatalyst for photocatalytic water oxidation with visible light. J Mater Chem A 3(35):17946–17950
Lin LW, Piao SQ, Choi Y et al (2022) Nanostructured transition metal nitrides as emerging electrocatalysts for water electrolysis: Status and challenges. EnergyChem 4(2):100072
Wang J, Xu F, Jin H et al (2017) Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications. Adv Mater 29(14):1605838
Cheng ZX, Qi WL, Pang CH et al (2021) Recent advances in transition metal nitride-based materials for photocatalytic applications. Adv Funct Mater 31(26):2100553
Yang Y, Zhou CY, Wang WJ et al (2021) Recent advances in application of transition metal phosphides for photocatalytic hydrogen production. Chem Eng J 405:126547
Ikeda Y, Lehmann TS, Widenmeyer M et al (2021) Crystal structure and phase stability of Co2N: a combined first-principles and experimental study. J Alloys Compd 854:156341
Yang Y, Zeng R, Xiong Y et al (2019) Cobalt-based nitride-core oxide-shell oxygen reduction electrocatalysts. J Am Chem Soc 141(49):19241–19245
Zhao X, Ke LQ, Wang CZ et al (2016) Metastable cobalt nitride structures with high magnetic anisotropy for rare-earth free magnets. Phys Chem Chem Phys 18(46):31680–31690
Chen PZ, Xu K, Tong Y et al (2016) Cobalt nitrides as a class of metallic electrocatalysts for the oxygen evolution reaction. Inorg Chem Front 3(2):236–242
Ma C, Hou PF, Kang P (2019) Electrocatalytic reduction of carbon dioxide to carbon monoxide using cobalt nitride. J Electrochem 25(4):467–476
Weng BC, Wei W, Yiliguma Y et al (2016) Bifunctional CoP and CoN porous nanocatalysts derived from ZIF-67 in situ grown on nanowire photoelectrodes for efficient photoelectrochemical water splitting and CO2 reduction. J Mater Chem A 4(40):15353–15360
Di J, Yan C, Handoko AD et al (2018) Ultrathin two-dimensional materials for photo- and electrocatalytic hydrogen evolution. Mater Today 21(7):749–770
Sun QQ, Wang M, Bao SJ et al (2016) Analysis of cobalt phosphide (CoP) nanorods designed for non-enzyme glucose detection. Analyst 141(1):256–260
Pei Y, Cheng Y, Chen JY et al (2018) Recent developments of transition metal phosphides as catalysts in the energy conversion field. J Mater Chem A 6(46):23220–23243
Pan Y, Lin Y, Chen YJ et al (2016) Cobalt phosphide-based electrocatalysts: synthesis and phase catalytic activity comparison for hydrogen evolution. J Mater Chem A 4(13):4745–4754
Theerthagiri J, Murthy AP, Lee SJ et al (2021) Recent progress on synthetic strategies and applications of transition metal phosphides in energy storage and conversion. Ceram Int 47(4):4404–4425
Feng LG, Xue HG (2017) Advances in transition-metal phosphide applications in electrochemical energy storage and catalysis. ChemElectroChem 4(1):20–34
Shi YM, Zhang B (2016) Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction. Chem Soc Rev 45(6):1529–1541
Zong Q, Liu CF, Yang H et al (2021) Tailoring nanostructured transition metal phosphides for high-performance hybrid supercapacitors. Nano Today 38:101201
Wang JM, Liu Z, Zheng YW et al (2017) Recent advances in cobalt phosphide based materials for energy-related applications. J Mater Chem A 5(44):22913–22932
Shi YM, Li MY, Yu YF et al (2020) Recent advances in nanostructured transition metal phosphides: synthesis and energy-related applications. Energy Environ Sci 13(12):4564–4582
Grosvenor AP, Wik SD, Cavell RG et al (2005) Examination of the bonding in binary transition-metal monophosphides MP (M = Cr, Mn, Fe, Co) by X-ray photoelectron spectroscopy. Inorg Chem 44(24):8988–8998
Qiao BT, Wang AQ, Yang XF et al (2011) Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat Chem 3(8):634–641
Liu JY (2017) Catalysis by supported single metal atoms. ACS Catal 7(1):34–59
Sun LL, Han L, Huang JT et al (2022) Single-atom catalysts for photocatalytic hydrogen evolution: a review. Int J Hydrog Energy 47(40):17583–17599
Ju L, Tan X, Mao X et al (2021) Controllable CO2 electrocatalytic reduction via ferroelectric switching on single atom anchored In2Se3 monolayer. Nat Commun 12(1):5128
Zhao CM, Wang Y, Li ZJ et al (2019) Solid-diffusion synthesis of single-atom catalysts directly from bulk metal for efficient CO2 reduction. Joule 3(2):584–594
Liu JY, Kong X, Zheng LR et al (2020) Rare earth single-atom catalysts for nitrogen and carbon dioxide reduction. ACS Nano 14(1):1093–1101
Sun HL, Ma YF, Zhang QT et al (2021) Engineering the local coordination environment of single-atom catalysts and their applications in photocatalytic water splitting: a review. Trans Tianjin Univ 27(4):313–330
Ye X, Ma JG, Yu WG et al (2022) Construction of bifunctional single-atom catalysts on the optimized β-Mo2C surface for highly selective hydrogenation of CO2 into ethanol. J Energy Chem 67:184–192
Zhang T, Lin WB (2014) Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem Soc Rev 43(16):5982–5993
Liu JW, Chen LF, Cui H et al (2014) Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem Soc Rev 43(16):6011–6061
He HB, Li R, Yang ZH et al (2021) Preparation of MOFs and MOFs derived materials and their catalytic application in air pollution: a review. Catal Today 375:10–29
Alkhatib II, Garlisi C, Pagliaro M et al (2020) Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: a review of strategies and applications. Catal Today 340:209–224
Chen Y, Wang DK, Deng XY et al (2017) Metal–organic frameworks (MOFs) for photocatalytic CO2 reduction. Catal Sci Technol 7(21):4893–4904
Radwan A, Jin HH, He DP et al (2021) Design engineering, synthesis protocols, and energy applications of MOF-derived electrocatalysts. Nanomicro Lett 13(1):132
Bates ED, Mayton RD, Ntai I et al (2002) CO2 capture by a task-specific ionic liquid. J Am Chem Soc 124(6):926–927
Huang YJ, Cui GK, Zhao YL et al (2017) Preorganization and cooperation for highly efficient and reversible capture of low-concentration CO2 by ionic liquids. Angew Chem Int Ed Engl 56(43):13293–13297
Rosen BA, Salehi-Khojin A, Thorson MR et al (2011) Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 334(6056):643–644
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Nos. 21905049 and 22178057), the Natural Science Foundation of Fujian Province (Nos. 2020J01201 and 2021J01197), the Research Foundation of the Academy of Carbon Neutrality of Fujian Normal University (TZH2022-07), and the Award Program for Minjiang Scholar Professorship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Shen, L. & Yang, MQ. Cobalt-Based Cocatalysts for Photocatalytic CO2 Reduction. Trans. Tianjin Univ. 28, 506–532 (2022). https://doi.org/10.1007/s12209-022-00350-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-022-00350-x