Abstract
Artificial photosynthetic reduction of CO2 into valuable chemicals is one of the most promising approaches to solve the energy crisis and decreasing atmospheric CO2 emissions. However, the poor selectivity accompanied by the low activity of photocatalysts limits the development of photocatalytic CO2 reduction. Herein, inspired by the use of oxygen vacancy engineering to promote the adsorption and activation of CO2 molecules, we introduced oxygen vacancies in the representative barium titanate (BaTiO3) photocatalyst for photocatalytic CO2 reduction. We found that oxygen vacancies brought significant differences in the CO2 photoreduction activity and selectivity of BaTiO3. The intrinsic BaTiO3 showed a low photocatalytic activity with the dominant product of CO, whereas BaTiO3 with oxygen vacancies exhibited a tenfold improvement in photocatalytic activity, with a high selectivity of ~ 90% to CH4. We propose that the presence of oxygen vacancies promotes CO2 and H2O adsorption onto the BaTiO3 surface and also improves the separation and transfer of photogenerated carriers, thereby boosting the photocatalytic CO2 reduction to CH4. This work highlights the essential role of oxygen vacancies in tuning the selectivity of photocatalytic reduction of CO2 into valuable chemicals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of the economy and society, the massive combustion of fossil fuels has resulted in a rapid increase in CO2 concentration in the atmosphere, leading to severe climate change [1,2,3]. The utilization of solar energy to achieve photocatalytic conversion of CO2 into carbonaceous fuels is one of the most beneficial approaches to solve the above issue [4, 5]. However, CO2 photoreduction with high performance and selectivity is challenging because the inert nature of CO2 leads to difficult activation. Furthermore, the process from the activated intermediate to the reactant involves complex electron and proton transfer kinetically [6, 7]. Endeavors in photocatalytic CO2 reduction since 1978 [8] involved the development of strategies including cocatalyst loading, surface modulation, and facet tailoring to modify the electronic and surface properties, such as light excitation [9], charge separation [10, 11], CO2 adsorption and activation [12,13,14,15], surface reactive sites [16,17,18], and intermediates [19]. Although the diverse products of photocatalytic CO2 reduction (including carbon monoxide [20], methane [21,22,23,24], methanol [25] or even multi-carbon products [26,27,28]) have been reported, strategies to precisely tune the selectivity of photocatalytic CO2 reduction are still in their infancy.
Oxygen vacancies with relatively low formation energy on oxide surfaces have attacted many attentions [29, 30]. Oxygen vacancies were widely recognized to act as electron traps to promote the separation of photogenerated charges in photocatalysis [31,32,33,34]. In addition, the local electron enrichment caused by the appearance of oxygen vacancies changes the surface chemical environment of the photocatalyst and promotes the adsorption and activation of CO2 in the photocatalytic CO2 reduction reaction [35,36,37,38,39]. Previously, we realized selective CO2 photoreduction to CO on the oxygen vacancy-involved hexagonal WO3. Besides, the in situ-generated oxygen vacancy lowers the CO2 activation barrier and promotes the photocatalytic CO2 reduction on WO3 [40]. It was also reported that an oxygen vacancy has a pinning effect on the ferroelectric monodomain of the material after corona polarization, providing a continuous driving force for the separation and migration of photogenerated charge carriers [41]. However, the main function of oxygen vacancies in most studies is considered to improve the adsorption and activation of CO2, with a lack of consideration of how oxygen vacancies affect the CO2 reduction pathway to tune the selectivity of products.
Herein, taking barium titanate (BaTiO3) as a prototype, we fabricated a series of BaTiO3 with tunable concentrations of oxygen vacancies by NaBH4 reduction for photocatalytic CO2 reduction. The BaTiO3 photocatalysts with oxygen vacancies showed a dramatic change in activity and selectivity of photocatalytic CO2 reduction. Further investigations indicated that the oxygen vacancies not only changes the surface and electronic nature of the BaTiO3, but also promotes the adsorption of CO2 and H2O molecules, which contribute to the controllable selectivity of the reduction of CO2 from CO to CH4. This work will be beneficial in fabricating the heterogeneous photocatalysts to tune the product distribution in photocatalytic CO2 reduction into chemicals.
Experimental
Synthesis of Tetragonal BaTiO3
BaTiO3 was synthesized using a reported sol–gel method [42]. All the chemicals were analytically pure without purification. 0.01 mol barium acetate was dissolved in 3 mL of glacial acetic acid, heated to 353 K, and then 5 mL ethylene glycol methyl ether was added to form a particle-free viscous dispersion. 5.14 mL glacial acetic acid, 1.84 mL acetylacetone, 0.71 mL formamide, and 0.81 mL deionized (DI) water were added to the above dispersion and then vigorously stirred for 10 min (Marked as solution A). Solution B consisted of 0.01 mol of tetrabutyl titanate with 10 mL ethylene glycol methyl ether and 2 mL acetylacetone as the solvent. The solutions A and B were quickly mixed to form solution C. Solution C was stirred in the air for 5 h and then dried in an oven at 453 K for 24 h to form a red gel. The gel was ground and then further heated in a muffle oven to form BaTiO3 nanocrystals.
BaTiO3 with oxygen vacancies was synthesized by molten salt treatment with adding NaBH4. 0.15 g of barium titanate, and 0.35 g of sodium borohydride were thoroughly grounded in a glove box, and then the mixture was heated in a tube furnace with a heating rate of 25 K/min to 598–698 K for 90 min in Ar flow of 200 mL/min. After the powder was cooled to room temperature, the sample was firstly washed with a stoichiometric amount of 3 mol/L hydrochloric acids and then washed with hot water to ensure that the boric acid was completely removed.
Characterization of the Catalyst
The morphology of the catalysts was characterized by scanning electron microscopy (Quanta 200 FEG, FEI) and high-resolution transmission electron microscopy (TECNAI G2 F30). The phase and crystallinity of the sample were determined via a Bruker D2 Phaser powder diffractometer using Cu Kα radiation ranging from 5° to 80°. The UV–visible diffuse reflectance experiment was carried out with a quartz cell using a UV–visible spectrophotometer (JASCO V-650). The EPR results were recorded by an electron paramagnetic resonance spectrometer (Bruker A200). A 325 nm-wavelength laser was selected as the Raman inlet light, and the experiments were performed using a Princeton Instruments Acton SpectraPro SP-2500 spectrometer.
Photocatalytic CO2 Performance Measurement
Photocatalytic CO2 reduction measurement was carried out in a homemade quartz reactor (Fig. S1). 50 mg catalyst was dispersed uniformly by ultrasonic agitation in 5 mL of saturated KHCO3 solution. Afterward, high-purity CO2 was purged into the reactor through the solution for 30 min. The reactor was kept gastight during the reaction. A 300 W Xe lamp was used as the light source. At regular intervals, the gas sample was withdrawn by syringe, and then analyzed by GC (Tianmei, 7900, 5A molecular sieve column with methane reformer and FID detector). For the isotope labeling experiments, the feed gas was replaced by 13CO2, and the gas product was analyzed by GC–MS.
Electrochemical Measurement
Electrodes were prepared by the drop coating method, with 5 mg catalyst dispersed into 5 mL of Nafion ethanol solution (0.25 wt%). After vigorous stirring, 1 mL of solution was added dropwise onto a 2 × 1 cm FTO substrate, and then the electrode was dried at 453 K. A saturated calomel electrode and 0.5 mol/L sodium sulfite solution were chosen as the reference electrode and the electrolyte, respectively.
In Situ FTIR Experiments
In situ FTIR experiments were carried out on a Nicolet 380 spectrometer with a TRS-20 MHz detector. A homemade quartz cell was utilized for placing the sample wafer. Before FTIR experiment, the catalyst was pre-heated at 393 K for 2 h. Ar, CO2, and CO2 purged through water were introduced into the cell sequentially. The signal was collected when it became stable. The sample was then irradiated by a 300 W Xe lamp after the sample reached a steady state in water vapor and CO2 atmosphere. The signal was collected once light irradiation was introduced into the cell.
Results and Discussion
BaTiO3 is a typical ferroelectric semiconductor used in photocatalysis. Despite reports that introducing oxygen vacancies in perovskite BaTiO3 could promote photocatalytic N2 reduction to NH3 [42], few studies have focused on the effect of oxygen vacancies in BaTiO3 for CO2 reduction. The BaTiO3 crystal consists an ABO3 unit cell (Fig. 1a), in which the oxygen atom can be removed by chemical reduction [43], resulting in the creation of an oxygen vacancy on the surface, making it possible to tune the oxygen vacancy gradient. Tetragonal BaTiO3 nanocrystals was synthesized as previously reported [42]. X-ray diffraction patterns of the as-prepared BaTiO3 are shown in Fig. 1b. All the diffraction peaks can be indexed to tetragonal BaTiO3 (JCPSB No.05-0626). Note that there is no significant peak splitting at 2θ = 45°, indicating that no cubic or mixture structure was present. TEM images show relatively regular nanocrystal features with a particle size of 50–100 nm (Fig. 1c). High-resolution TEM image in Fig. 1d shows that the length of the lattice spacing is 4.04 Å, ascribed to the typical (001) plane of the tetragonal BaTiO3, which is consistent with the XRD patterns.
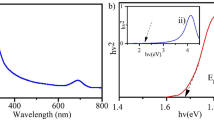
BaTiO3 with oxygen vacancies gradient was obtained by molten salt treatment with adding NaBH4 under different temperatures. The XRD patterns in Fig. 2a show that the intensity of the main diffraction peak displays no obvious change when the temperature was below 673 K, whereas BaTiO3 treated at 698 K displayed a wider full width at half maximum (FWHM) and weaker peak intensity, demonstrating a slight decrease in crystallinity. No additional diffraction peak appears, even when the temperature is higher than the Curie temperature. The morphology of BaTiO3 can be maintained after the treatment under different temperatures (Fig. S2). UV–visible diffusion reflection spectroscopy was then carried out to study the absorbance difference. As shown in Fig. 2b, as the temperature increases, the absorption of the BaTiO3 in the visible light region gradually increases. It is generally accepted that the increased absorption intensity in the visible light region can be ascribed as the increase in low-valent metal species, which confirms the gradual increase of oxygen vacancies in the BiTiO3. It has been demonstrated that the loss of oxygen in the lattice would induce the slight decrease in cell volume and cell parameters [37]. The presence of oxygen vacancies was also verified by the electron paramagnetic resonance (EPR) spectra, as shown in Fig. 2c. An obvious signal appeared at g = 2.002, corresponding to the signal of free electrons captured by holes, was observed, and the intensity increased gradually with the increase in the reduction temperature. The intensity of the signal displays a consistent trend versus the reduction temperature, illustrating that the concentration of oxygen vacancies increases with the reduction temperature. Analysis of Raman spectra was carried out to verify the structure evolution of BiTiO3 (Fig. 2d). A typical tetragonal phase of BaTiO3 for the intrinsic BiTiO3 exhibits the characteristic sharp peak at 270 cm−1 and broad bands at 517 cm−1 and 705 cm−1 [44]. Note that when the BaTiO3 was reduction treated at 598 K, the corresponding Raman peak intensity decreases, and the FWHM increases. Further increasing the reduction temperature to 623 K, the characteristic peak corresponding to the BaTiO3 disappears, which may be due to the destruction of the surface structure by the removal of oxygen and forming an amorphous layer, which is consistent with the previously reported results [37, 45, 46]. The above results demonstrate that the BaTiO3 with different oxygen vacancy concentrations can be obtained by molten salt treatment in the presence of NaBH4 at different temperatures.
Photocatalytic CO2 reduction performance was examined in the CO2 gas saturated KHCO3 solution. As shown in Fig. 3a, b, both intrinsic and oxygen-vacancy-involved BaTiO3 samples enable the realization of the photocatalytic CO2 reaction but exhibit a significant difference in photocatalytic activity and selectivity. For intrinsic BaTiO3, the only product was CO with no CH4 was detected. With the increase in the reducing temperature to create oxygen vacancies in the BaTiO3, the amount of CO was reduced, whereas CH4 was detected in the product and the CH4 amount gradually increased. Blank experiments, including without introduction of BaTiO3 or without light irradiation, were also performed to confirm the produced CO and CH4 were indeed originated from the photocatalytic CO2 reduction on BaTiO3 (Fig. S4). The CH4 selectivity of BaTiO3 reduced at 698 K in 2 h was measured to be ~ 90%. A direct comparison of real-time gas chromatograms was also shown in Fig. S5. When the reduction temperature exceeds 698 K, the obtained sample is a flammable solid with a black metallic luster. The BiTiO3 catalyst reduced at 673 K exhibited a better photocatalytic activity of CO2 reduction to CO, whereas the BaTiO3 reduced at 698 K was optimized to be superior to others toward CH4 and overall carbonaceous products (Fig. S6). Photocatalytic CO2 reduction of BaTiO3 reduced at 698 K in pure water were also conducted (Fig. S7). Given the low solubility of CO2 in water, the activity in water was found to be far below that in KHCO3-CO2 saturated solution.
Photocatalytic performance of the intrinsic and oxygen vacancy-involved BaTiO3. Time course of a CO and b CH4 evolution for intrinsic and oxygen vacancy-involved BaTiO3 under UV–visible illumination in saturated CO2-KHCO3 solution. Mass spectra extracted from GC–MS analysis of the products c CO and d CH4 with isotope labeling using 13CO2. e Long-time course reaction performance for BaTiO3 reduced at 698 K. f CO and CH4 evolution comparison for BaTiO3 reduced at 698 K and that re-reduced at 698 K. The temperatures marked in the figures refer to the sample with the corresponding reduction temperature
Isotope labeling experiments with 13CO2 as the reactant were carried out to determine the source of the product. As shown in Fig. 3c, d, when the reactant is 12CO2, only a single peak appears at the position of m/z = 28, which corresponds to the reduction product of CO from 12CO2. When the reactant was replaced by 13CO2, a new peak appeared at the position of m/z = 29, which proved that CO is the product of CO2 reduction. Moreover, when the reactant changed from 12CO2 to 13CO2, the peak position in the GC–MS spectrum changed from m/z = 16 to 17, which verified that the product of CH4 comes from CO2 photoreduction rather than possible contaminants. To determine the stability of the BaTiO3 catalyst, as shown in Fig. 3e, f, the time curve and cycling reaction of the BaTiO3 catalyst were conducted. The photocatalytic activity of the BaTiO3 catalyst gradually decreases after long-term reaction. When the BaTiO3 was retreated by NaBH4 reduction, the photocatalytic performance of the catalyst significantly decreases, which may be due to the over-reduction of the catalyst, leading to the collapse of the surface structure. Although the long-term stability of the BaTiO3 still requires improvement, the selectivity of CO2 photoreduction toward CH4 was maintained after cycling reactions for a long period.
To examine the presence of oxygen vacancies on the selectivity in photocatalytic CO2 reduction, CO2 adsorption and electrochemical measurements were carried out. As shown in Figs. 4a and S8, when the reduction temperature was tuned between 598 and 648 K, the CO2 adsorption capacity abnormally decreased, which may be caused by the formation of a metastable structure that destroyed the original CO2 adsorption sites. When the reduction temperature reaches 673 K, the CO2 adsorption capacity dramatically increases. Note that the CO2 adsorption changes is consistent with the trend of CO2 photoreduction performance, indicating that the oxygen vacancies may change the surface nature of BaTiO3, subsequently influencing the CO2 adsorption and activation and causing the differences in CO2 photoreduction. Furthermore, it is recognized that the CO2 photoreduction toward CH4 involves eight electrons and eight protons transfer process [47], for which photogenerated carrier transport also plays a critical role in the whole reaction process. As shown in Fig. 4b, the BaTiO3 reduced at 698 K with oxygen vacancies exhibits an enhanced photoelectric response compared to intrinsic BaTiO3. In addition, the electrochemical impedance spectrum (EIS) measurements demonstrate that the BaTiO3 reduced at 698 K possesses the smallest arc radius on resistance, indicating a better interfacial electron transfer on the BaTiO3 with oxygen vacancies.
Electronic and surface characterization of intrinsic and oxygen vacancy-involved BaTiO3. a CO2 adsorption spectra of intrinsic and oxygen vacancy-involved BaTiO3. b Photocurrent density of intrinsic and oxygen vacancy-involved BaTiO3 under light irradiation. c EIS spectra of intrinsic and oxygen vacancy-involved BaTiO3. d Contact angle of the intrinsic and oxygen vacanvy-involved BaTiO3. The temperatures marked in the figures refer to the sample with the corresponding reduction temperature
The selectivity of CO2 reduction is not only correlated with the CO2 adsorption and electron migration, but also related to the adsorption capacity of H2O molecules. We further measured the contact angle for water molecules on the intrinsic and oxygen vacancy-involved BaTiO3. As shown in Fig. 4d, the contact angle to water molecules of BaTiO3 displays a trend of increasing first and then declining, and the BaTiO3 with the reduction temperature of 623 K exhibits the largest contact angle to the water molecules. As the reduction temperature gradually increased, the BiTiO3 surface became hydrophilic gradually, accompanied by the gradual generation of CH4. The results indicates that it is essential for the BiTiO3 photocatalyst to adsorb water molecules for subsequent proton-coupled electron transfer to produce CH4.
To further elucidate the photocatalytic CO2 reduction mechanism, in situ Fourier transform infrared spectrum (FTIR) measurements were performed. For intrinsic BaTiO3, as shown in Fig. 5a, a new peak attributed to HCO3− appeared at 1760 cm−1. When CO2 and water vapor were introduced into the reactor, following irradiation, the intensity of the peak at 1640 cm−1 ascribed to OH− dramatically increased, indicating strong adsorption of water molecules. The peak at 1760 cm−1 vanished, and a new peak attributed to CO emerged, demonstrating that the CO2 photoreduction occurs on the intrinsic BaTiO3 with an ordinary process from CO2 → HCO3− → CO, as we previously reported [34]. To further elaborate on the reaction pathway, FTIR experiment was carried out on the oxygen vacancy-involved BiTiO3 under the same condition. It was found that a weak peak corresponding to the CH3O− was formed around 1160 cm−1, which was considered to be an intermediate in the “Carbene Pathway” [48].
It is widely recognized that CO2 photoreduction to CH4 involves a process of CO2 adsorption to charge transfer and subsequent reaction with adsorbed CO2. Therefore, based on the results above, CO2 and water adsorption directly determine the amount of reactants, which further determines the overall reduction of CO2 to carbonaceous products. Furthermore, the selectivity of CO2 toward CH4 is directly related to the electron concentration on the surface. As shown in Scheme 1, by rational design and treatment of the BaTiO3 catalyst, oxygen vacancies with a proper concentration would not only facilitate CO2 adsorption and activation but also provide sufficient photogenerated carriers to the surface for water oxidation and subsequent proton coupling electron transfer to produce CH4.
Conclusion
In summary, we obtained BaTiO3 with different oxygen vacancy concentrations using the molten salt treatment in the presence of NaBH4 and found that the presence of oxygen vacancies significantly affects the activity and selectivity of photocatalytic CO2 reduction. Intrinsic BaTiO3 shows a low photocatalytic activity with the dominant product of CO, whereas BaTiO3 with oxygen vacancies exhibited a tenfold improvement in photocatalytic activity with a high selectivity of ~ 90% toward CH4. We proposed that the presence of oxygen vacancies benefits CO2 and H2O adsorption onto the BaTiO3 surface and also improves the separation and transfer of photogenerated carriers, thereby boosting the selective photocatalytic CO2 reduction to methane. A proper oxygen vacancy concentration would optimize the surface chemical and electronic structure to further control the surface reactant concentration, leading to a relatively high concentration and selectivity. Our work highlights the essential role of oxygen vacancies in tuning the selectivity of photocatalytic CO2 reduction into valuable chemicals.
References
Navarro-Jaén S, Virginie M, Bonin J et al (2021) Highlights and challenges in the selective reduction of carbon dioxide to methanol. Nat Rev Chem 5(8):564–579
Fu JW, Jiang KX, Qiu XQ et al (2020) Product selectivity of photocatalytic CO2 reduction reactions. Mater Today 32:222–243
Nahar S, Zain MFM, Kadhum AAH et al (2017) Advances in photocatalytic CO2 reduction with water: a review. Materials 10(6):629
Zhang GG, Li GS, Heil T et al (2019) Tailoring the grain boundary chemistry of polymeric carbon nitride for enhanced solar hydrogen production and CO2 reduction. Angew Chem Int Ed Engl 58(11):3433–3437
Ran J, Jaroniec M, Qiao SZ (2018) Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities. Adv Mater 30(7):1704649
Chang XX, Wang T, Gong JL (2016) CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ Sci 9(7):2177–2196
Wagner A, Sahm CD, Reisner E (2020) Towards molecular understanding of local chemical environment effects in electro- and photocatalytic CO2 reduction. Nat Catal 3(10):775–786
Halmann M (1978) Photoelectrochemical reduction of aqueous carbon dioxide on p-type gallium phosphide in liquid junction solar cells. Nature 275(5676):115–116
Chen XB, Liu L, Yu PY et al (2011) Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331(6018):746–750
Chen F, Ma TY, Zhang TR et al (2021) Atomic-level charge separation strategies in semiconductor-based photocatalysts. Adv Mater 33(10):2005256
Dong CC, Ji JH, Yang Z et al (2019) Research progress of photocatalysis based on highly dispersed titanium in mesoporous SiO2. Chin Chem Lett 30(4):853–862
Huo HL, Liu D, Feng H et al (2020) Double-shelled Cu2O/MnOx mesoporous hollow structure for CO2 photoreduction with enhanced stability and activity. Nanoscale 12(26):13912–13917
Qiu CH, Bai S, Cao WJ et al (2020) Tunable syngas synthesis from photocatalytic CO2 reduction under visible-light irradiation by interfacial engineering. Trans Tianjin Univ 26(5):352–361
Zhao ZJ, Liu ZL, Zhu ZX et al (2021) Ultrathin zinc selenide nanosheet-based intercalation hybrid coupled with CdSe quantum dots showing enhanced photocatalytic CO2 reduction. Chin Chem Lett 32(8):2474–2478
Yoshino S, Takayama T, Yamaguchi Y et al (2022) CO2 reduction using water as an electron donor over heterogeneous photocatalysts aiming at artificial photosynthesis. Acc Chem Res 55(7):966–977
Collado L, Reñones P, Fermoso J et al (2022) The role of the surface acidic/basic centers and redox sites on TiO2 in the photocatalytic CO2 reduction. Appl Catal B Environ 303:120931
Lan ZA, Wang XC (2015) ChemInform abstract: merging surface organometallic chemistry with graphitic carbon nitride photocatalysis for CO2 photofixation. ChemCatChem 7(9):1422–1423
Ye LQ, Wu D, Chu KH et al (2016) Phosphorylation of g-C3N4 for enhanced photocatalytic CO2 reduction. Chem Eng J 304:376–383
Liu Q, Cheng H, Chen TX et al (2022) Regulating the *OCCHO intermediate pathway towards highly selective photocatalytic CO2 reduction to CH3CHO over locally crystallized carbon nitride. Energy Environ Sci 15(1):225–233
Xi GC, Ouyang SX, Li P et al (2012) Ultrathin W18O49 nanowires with diameters below 1 nm: synthesis, near-infrared absorption, photoluminescence, and photochemical reduction of carbon dioxide. Angew Chem Int Ed Engl 51(10):2395–2399
Tahir M, Amin NS (2015) Indium-doped TiO2 nanoparticles for photocatalytic CO2 reduction with H2O vapors to CH4. Appl Catal B Environ 162:98–109
Dimitrijevic NM, Vijayan BK, Poluektov OG et al (2011) Role of water and carbonates in photocatalytic transformation of CO2 to CH4 on titania. J Am Chem Soc 133(11):3964–3971
Wang Y, Zhang ZZ, Zhang LN et al (2018) Visible-light driven overall conversion of CO2 and H2O to CH4 and O2 on 3D-SiC@2D-MoS2 heterostructure. J Am Chem Soc 140(44):14595–14598
Li XD, Sun YF, Xu JQ et al (2019) Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat Energy 4(8):690–699
Zeng GT, Qiu J, Li Z et al (2014) CO2 reduction to methanol on TiO2-passivated GaP photocatalysts. ACS Catal 4(10):3512–3516
Dai WL, Xu H, Yu JJ et al (2015) Photocatalytic reduction of CO2 into methanol and ethanol over conducting polymers modified Bi2WO6 microspheres under visible light. Appl Surf Sci 356:173–180
Liu YY, Huang BB, Dai Y et al (2009) Selective ethanol formation from photocatalytic reduction of carbon dioxide in water with BiVO4 photocatalyst. Catal Commun 11(3):210–213
Sun SM, Watanabe M, Wu J et al (2018) Ultrathin WO3·0.33H2O nanotubes for CO2 photoreduction to acetate with high selectivity. J Am Chem Soc 140(20):6474–6482
Li H, Li J, Ai ZH et al (2018) Oxygen vacancy-mediated photocatalysis of BiOCl: reactivity, selectivity, and perspectives. Angew Chem Int Ed Engl 57(1):122–138
Wang JL, Kang SH, Zhu XG et al (2021) Highly ordered Nb2O5 nanochannel film with rich oxygen vacancies for electrocatalytic N2 reduction: inactivation and regeneration of electrode. Chin Chem Lett 32(9):2833–2836
Yang S, Halliburton LE, Manivannan A et al (2009) Photoinduced electron paramagnetic resonance study of electron traps in TiO2 crystals: oxygen vacancies and Ti3+ ions. Appl Phys Lett 94(16):162114
Lei FC, Sun YF, Liu KT et al (2014) Oxygen vacancies confined in ultrathin indium oxide porous sheets for promoted visible-light water splitting. J Am Chem Soc 136(19):6826–6829
Liao JZ, Li KL, Ma H et al (2020) Oxygen vacancies on the BiOCl surface promoted photocatalytic complete NO oxidation via superoxide radicals. Chin Chem Lett 31(10):2737–2741
Ma ZY, Li PH, Ye LQ et al (2017) Oxygen vacancies induced exciton dissociation of flexible BiOCl nanosheets for effective photocatalytic CO2 conversion. J Mater Chem A 5(47):24995–25004
Wu SQ, Wang JB, Li QC et al (2021) Bi/BiOCl nanosheets enriched with oxygen vacancies to enhance photocatalytic CO2 reduction. Trans Tianjin Univ 27(2):155–164
Li Q, Liu YN, Wan Z et al (2022) Microwave-assisted synthesis of oxygen vacancy associated TiO2 for efficient photocatalytic nitrate reduction. Chin Chem Lett 33(8):3835–3841
Jiang LS, Li Y, Wu XY et al (2021) Rich oxygen vacancies mediated bismuth oxysulfide crystals towards photocatalytic CO2-to-CH4 conversion. Sci China Mater 64(9):2230–2241
Ji YF, Luo Y (2016) New mechanism for photocatalytic reduction of CO2 on the anatase TiO2 (101) surface: the essential role of oxygen vacancy. J Am Chem Soc 138(49):15896–15902
Ye LQ, Deng Y, Wang L et al (2019) Bismuth-based photocatalysts for solar photocatalytic carbon dioxide conversion. ChemSusChem 12(16):3671–3701
Wang Y, Liu RZ, Shi M et al (2022) Photo-induced carbon dioxide reduction on hexagonal tungsten oxide via an oxygen vacancies-involved process. Chin Chem Lett. https://doi.org/10.1016/j.cclet.2022.02.006
Yu HJ, Chen F, Li XW et al (2021) Synergy of ferroelectric polarization and oxygen vacancy to promote CO2 photoreduction. Nat Commun 12(1):1–10
Zhao Z, Wang DD, Gao R et al (2021) Magnetic-field-stimulated efficient photocatalytic N2 fixation over defective BaTiO3 perovskites. Angew Chem Int Ed Engl 60(21):11910–11918
Zhao ZY, Li GR, Wang Z et al (2019) Black BaTiO3 as multifunctional sulfur immobilizer for superior lithium sulfur batteries. J Power Sources 434:226729
Hayashi H, Nakamura T, Ebina T (2013) In-situ Raman spectroscopy of BaTiO3 particles for tetragonal-cubic transformation. J Phys Chem Solids 74(7):957–962
Guo M, Lu JQ, Wu YN et al (2011) UV and visible Raman studies of oxygen vacancies in rare-earth-doped ceria. Langmuir 27(7):3872–3877
Wang YT, Cai JM, Wu MQ et al (2018) Rational construction of oxygen vacancies onto tungsten trioxide to improve visible light photocatalytic water oxidation reaction. Appl Catal B Environ 239:398–407
Kovacic Z, Likozar B, Hus M (2020) Photocatalytic CO2 reduction: a review of ab initio mechanism, kinetics, and multiscale modeling simulations. ACS Catal 10(24):14984–15007
Tan SS, Zou LD, Hu E (2008) Kinetic modelling for photosynthesis of hydrogen and methane through catalytic reduction of carbon dioxide with water vapour. Catal Today 131(1–4):125–129
Acknowledgements
The work was supported by the National Key Research and Development Program of China (2021YFA1502300), National Natural Science Foundation of China (Nos. 22090033), Youth Innovation Promotion Association of the Chinese Academy of Sciences and the National Youth Talent Support Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Zhang, C. & Li, R. Modulating the Selectivity of Photocatalytic CO2 Reduction in Barium Titanate by Introducing Oxygen Vacancies. Trans. Tianjin Univ. 28, 227–235 (2022). https://doi.org/10.1007/s12209-022-00334-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-022-00334-x