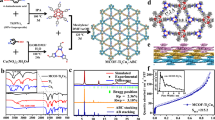
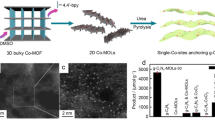
Abstract
Photocatalytic water splitting and carbon dioxide photoreduction are considered effective strategies for alleviating the energy crisis and environmental pollution. Polynuclear metal-oxo clusters possess excellent electron storage/release ability and unique catalytic properties via intermetallic synergy, which enables them with great potential in environmentally friendly photosynthesis. Importantly, metal-oxo clusters with precise structure can not only act as high-efficiency catalysts but also provide well-defined structural models for exploring structure–activity relationships. In this review, we systematically summarize recent progress in the catalytic application of polynuclear metal-oxo clusters, including polyoxometalate clusters, low-cost transition metal clusters, and metal-oxo-cluster-based metal–organic frameworks for water splitting and CO2 reduction. Furthermore, we discuss the challenges and solutions to the problems of polynuclear metal-oxo clusters in photocatalysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The unrestrained consumption of fossil fuels has dramatically increased the concentration of carbon dioxide (CO2) in the atmosphere and exacerbated the worldwide energy crisis and greenhouse effect [1,2,3]. Photocatalytic water splitting and CO2 reduction are considered effective strategies for alleviating the energy crisis and excessive carbon dioxide emissions [4]. Reducing the reaction activation energy and improving the reaction rate is crucial for water splitting into hydrogen and carbon dioxide photoreduction. Photocatalytic overall water splitting driven by solar energy can simultaneously generate H2 and O2, and H2 is an ideal energy carrier to replace traditional fossil fuels because of its clean combustion characteristics and high energy density [5,6,7]. Photoreduction of CO2 into value-added carbon-based products, including hydrocarbon fuels (e.g., CH4, C2H4, and C2H6) or chemicals (e.g., HCOOH, CH3OH, and CH3COOH), can push the end products of fossil fuels back into the carbon cycle [8,9,10,11]. To date, a variety of inorganic semiconductors have been studied for efficient energy conversion [12], but their indistinct active sites and imprecise microenvironments with complicated structures have impeded the efforts to reveal mechanistic insights into the energy conversion process.
Molecular clusters composed of multimetal centers with adjustable composition are widely used as photocatalysts with excellent catalytic ability to further provide mechanistic insight at the molecular level because of their well-defined structure [13,14,15]. Among these cluster catalysts, polyoxometalates (POMs) are a large class of metal-oxo clusters composed of the earth-abundant elements V, Nb, Ta, Mo, and W, which represent a tremendous range of crystalline clusters with abundant physical and chemical properties [16,17,18]. Recently, POMs have been widely used in photocatalytic water splitting to produce hydrogen and oxygen, as well as carbon dioxide photoreduction. Moreover, various rare earth metal clusters have been widely investigated because of their good performance and extensively tunable catalytic properties in photocatalysis [19]. Recently, low-cost transition metals with unfilled valence 3d orbitals have been constructed into transition metal clusters with tens or hundreds of atoms, which may achieve excellent performance for photocatalysis via an interatomic coupling or synergistic excitation [20, 21]. Recently, transition metal clusters have made great advances in photocatalysis. In this field, polynuclear metal-oxo clusters possess excellent electron storage/release ability and unique catalytic properties via intermetallic synergy, which enables them with great potential in environmentally friendly photosynthesis. Importantly, metal-oxo clusters with precise structure can not only act as high-efficiency catalysts but also provide well-defined structural models for exploring structure–activity relationships. This review summarizes the important application progress of cluster-based catalysts in energy conversion, such as water splitting and carbon dioxide reduction. Further, the challenges and solutions to the problems of polynuclear metal-oxo clusters in catalysis are also discussed.
POM-Based Photocatalysts for Energy Conversion
Photocatalytic Water Splitting for Hydrogen Production
Under the double pressure of the energy crisis and environmental pollution, sustainable clean energy must be developed to reduce fossil fuel use [22]. For a long time, researchers searched for effective, low-cost, and sustainable ways to promote water splitting into hydrogen [23]. Since 1972, when photocatalytic water splitting on TiO2 electrodes was achieved [5], photocatalytic H2 evolution has been considered a promising approach to overcoming the energy crisis and environmental issues. However, the current photocatalysts for water splitting have disadvantages, such as a low H2 yield and the need for a precious metal as a cocatalyst or photosensitizer [24]. Therefore, developing a cheap and efficient photocatalyst for water splitting is desirable but remains very challenging.
Inspired by the photocatalytic H2 production on TiO2 in 1972, great progress has been achieved in recent decades by investigating metal oxide semiconductors (MOSs, such as TiO2, titanates, Ta2O5, and tantalates) as photocatalysts [25,26,27]. POMs, as an important subclass of metal-oxo clusters, possess inherent redox ability and semiconductor-like characteristics (Scheme 1).
POM Used as a Photocatalyst Directly
In 2011, Feng’s group [28] reported a new heteropolyoxonibate, [Nb2O2(H2O)2][SiNb12O40]10−, with photocatalytic water splitting activity, and its photocatalytic activity is substantially higher than that of [Nb2O2][SiNb12O40]10−. This work revealed that the coordinating water molecule to the bridging Nb5+ center can lead to highly unsymmetrical seven-coordinated Nb5+ sites, which contribute greatly to the enhanced photocatalytic activity for H2 production. Subsequently, Wang et al. [29] synthesized three polyoxoniobate clusters, {Nb24O72}, {Nb32O96}, and {K12Nb96O288}, which can be used as photocatalysts for H2 evolution with CoIII(dmgH)2pyCl as a cocatalyst under UV irradiation. At the same time, Liu et al. [30] reported {Ta12}/{Ta16} cluster-containing polytantalotungstates with remarkable photocatalytic H2 evolution activity. The high activity of {Ta12}-based POMs can be further rationalized by the presence of distorted heptacoordinated Ta atoms as a TaO7 pentagonal bipyramid. Furthermore, a series of high-nuclear spin nickel cluster-containing POMs were designed and synthesized with the lacunary POM as the coordination ligands. Introducing transition metal clusters improves their catalytic performance for hydrogen production under visible light irradiation with the assistance of noble metal photosensitizers [31, 32]. Recently, Lv’s group [33, 34] introduced transition metals such as manganese and iron into the lacunary Keggin polyoxometalate to achieve photocatalytic H2 evolution.
POM@photosensitizers Assembled and Used as Photocatalysts
However, traditional POMs often display light absorption in the ultraviolet region because of their large bandgap energy, which severely limits the improvement of the photocatalytic efficiency of the hydrogen evolution reaction (HER) [35,36,37]. Therefore, researchers have developed several strategies to broaden the spectral response range of catalysts. First, Lin et al. [24] constructed a charge-assisted hybrid POM@photosensitizers system in which a [P2W18O62]6− molecule as the electron acceptor was encapsulated in a [Ru(bpy)3]2+-based MOF for the photocatalytic HER (Fig. 1). Its HER performance can be much enhanced compared to that of the homogeneous catalytic system under visible light irradiation because of a fast multielectron injection from the photoactive framework to the polyoxoanion. Subsequently, they synthesized another [Ni4(H2O)2(PW9O34)2]10−@photosensitizers composite by encapsulating a Ni-containing polyoxoanion of [Ni4(H2O)2(PW9O34)2]10− into [Ir(ppy)2(bpy)]+-based phosphorescent UiO-MOFs [38]. The [Ni4(H2O)2(PW9O34)2]10−@[Ir(ppy)2(bpy)] composite exhibited efficient visible light-driven HER activity with a turnover number as high as 1476. A systematic study revealed that each [Ni4(H2O)2(PW9O34)2]10− anion was surrounded closely by multiple [Ir(ppy)2(bpy)]+ photosensitizers, which can facilitate facile multielectron transfer to contribute to the enhanced HER activity. Li et al. [39] prepared a supramolecular framework from a hexa-armed [Ru(bpy)3]2+-based precursor and cucurbituril, and the resulting supramolecular framework could adsorb Wells–Dawson-type POM [P2W18O62]6− to form photoactive POM@photosensitizer hybrids for catalytic hydrogen generation. Recently, Lv’s group [40] reported a facile and broad-spectrum impregnation method to construct two POM@MOF composites, Ni3PW10@NU-1000 and Ni3P2W16@NU-1000. In these composites, a tri-Ni-substituted Keggin-type polyoxoanion [Ni3(H2O)3PW10O39H2O]7− and a Wells–Dawson-type polyoxoanion [Ni3(OH)3(H2O)3P2W16O59]9− were incorporated into a mesoporous Zr-based metal–organic framework (NU-1000), respectively. Under the optimized conditions, the resulting POM@MOF composites can effectively catalyze hydrogen production with superior long-term stability and reusability, and a hydrogen evolution rate of 3482 and 13,051 μmol/(g h) can be achieved for Ni3PW10@NU-1000 and Ni3P2W16@NU-1000, respectively.

Reproduced with permission from Ref. [24]. Copyright © 2015, American Chemical Society
Synthesis of the POM@photosensitizer system via charge-assisted self-assembly and photocatalytic water splitting for hydrogen production.
Covalent Photosensitizer-POM Attached and Used as a Photocatalyst
Introducing a photosensitizer into the POM frameworks was considered an efficient method for obtaining visible light-active photocatalysts for water splitting. Zhang et al. [41] synthesized two W/Ta mixed-addendum nanoclusters grafted with TM-organic fragments, resulting in two UV, visible, and NIR light-active molecular clusters for water splitting. Carsten’s group [42] reported a new route to link an iridium photosensitizer to an Anderson-type hydrogen-evolution catalyst. This covalent dyad catalyzes the visible-light-driven HER and shows superior HER activity compared with the noncovalent reference. Subsequently, they further used a fully integrated photochemical molecular dyad consisting of a Ru photosensitizer covalently attached to a Wells–Dawson POM, which can act as an electron storage and hydrogen evolving catalyst. The molecular photosensitizer-POM dyad shows excellent chemical and photochemical stability [43].
POM-Semiconductor Hybrids Used as a Photocatalyst
Compared with traditional organometallic or pure organic molecular photosensitizers, semiconductor nanocrystals materials have received extensive attention in recent years for solar energy harvesting [44]. More importantly, the semiconductor nanocrystal-based photosensitizers generally exhibit ultrahigh photostability and broad visible light absorption properties. In 2013, Zhang's group [45] successfully synthesized the molecular self-assembled CdS QD-POM-Au nanohybrids. The tricomponent nanohybrids exhibited photocatalytic activity for hydrogen evolution through water splitting via visible light irradiation. Recently, photocatalytic hydrogen evolution in pure water was realized by Ding’s group [46]. CdS was used as the light-harvesting group, and CQDs were used as the electron acceptor and donor with [Ni4(H2O)2(PW9O34)2]10− as the catalyst. Under visible light irradiation (λ = 420 nm), this catalytic system exhibits good water splitting activity, with a H2 evolution rate of up to 145 μmol/(gcat·h). Lv’s group [47] coupled water-soluble CdSe QD light-absorbers with Ni-substituted polyoxometalate (Ni–POM) catalysts and an AA electron donor. The present CdSe + POM catalytic system exhibits superior and robust hydrogen production activity.
Photocatalytic Water Oxidation
Water oxidation for oxygen production represents a multielectron transfer process with high oxidation potential, which is considered the bottleneck in overall water splitting [48]. As a result, considerable effort has been devoted to developing water oxidation catalysts (WOCs) in recent decades [49, 50]. Among these heterogeneous and homogeneous catalysts, POMs represent a great subclass of WOCs that not only possess a well-defined structure but also can efficiently drive water oxidation.
In 2010, Hill et al. [51] reported a [Co4(H2O)2(PW9O34)2]10− POM comprising a Co4 core stabilized by oxidatively resistant polytungstate ligands, which can act as an efficient water oxidation catalyst sensitized by [Ru(bpy)3]2+. In 2013, Ding et al. [52] reported the Co-substituted Keggin POM K7[CoIIICoII(H2O)W11O39] for efficient visible light-driven O2 production and thermal catalytic water oxidation. In 2014, Zhang et al. [53] reported four all-inorganic, abundant-metal-based high-nuclear spin cobalt–phosphate-substituted POMs, which can be used as molecular catalysts for visible light-driven water oxidation. The Co4O4 cubane in the {Co16(PO4)4} cluster is structurally analogous to the [Mn3CaO4] core of the oxygen-evolving center in photosystem II (PSII). These four compounds were the first POM-based cobalt-phosphate-cluster molecular catalysts for visible light-driven water oxidation; thus, they can serve as a functional model of the oxygen-evolving catalysts. A systematic study first revealed that heteroatom regulation can realize the regulation of photocatalytic performance for the water oxidation of these POM clusters. Subsequently, three high-nuclear spin nickel clusters, {Ni12}, {Ni13}, and {Ni25}, were encapsulated in the lacunary of POMs via a similar synthetic process. These three compounds contain {Ni3O3} quasi-cubane or {Ni4O4} cubane units, which can be used for visible light-driven water oxidation [54]. These results provide all-inorganic polynuclear Co/Ni-based structural models for visible light-driven water oxidation. In 2014, Kortz’s group [55] reported a tetramanganese-substituted tungstosilicate [MnIII3MnIVO3(CH3COO)3(SiW9O34)]6− as the photocatalyst for water oxidation, which was composed of a mixed-valent MnIII3MnIVO3 Mn-oxo core to mimic the natural oxygen-evolving center (Mn4O5Ca), which has been observed in a Mn12-based POM [56]. In 2014, the water oxidation catalyst [(VIV5VV)O7(OCH3)12]− consisting of vanadium centers was reported [57], which opened the way to using non-expensive vanadium clusters for water oxidation in artificial photosynthesis. Then, Hill’s group [58] reported a homogeneous carbon-free cobalt-based water oxidation catalyst based on redox-active V-centered POM ligands, [Co4(H2O)2(VW9O34)2]10−. In 2018, Dolbecq and coworkers [59] immobilized the sandwich-type polyoxoanion [(PW9O34)2Co4(H2O)2]10− in the hexagonal channels of a ZrIV-porphyrinic MOF-545 hybrid framework (Fig. 2). The composite material exhibits high photocatalytic activity and good stability for visible light-driven water oxidation.

Reproduced with permission from Ref. [59]. Copyright © 2018, American Chemical Society
[(PW9O34)2Co4(H2O)2]@MOF-545 for photocatalytic water oxidation.
Photocatalytic CO2 Reduction
Global energy demands largely depend on the combustion of fossil fuels, including coal, petroleum, and natural gas [60]. As the main component of greenhouse gas, CO2 is a key product during fossil fuel combustion. The immense emission of CO2 has resulted in severe environmental issues, such as global warming and extreme weather [61]. As is well known, fossil fuels will remain a major energy source in the foreseeable future, and CO2 conversion into fuels represents a straightforward strategy for solving the energy crisis and environmental problems [62]. Therefore, exploring cluster catalysts for CO2 photoreduction has attracted wide attention, and great progress has been made in this field.
In 2011, Ronny et al. [63] decorated a phenanthroline ligand at the 5, 6-position of a 15-crown-5 ether, which was used to prepare a metal–organic POM hybrid complex ReI(L)(CO)3CH3CN-MHPW12O40 (L = 15-crown-5-phenanthroline, M = Na+, H3O+). In the presence of Pt/C, the POM moiety in ReI(L)(CO)3CH3CN-MHPW12O40 can oxidize H2 to produce protons and electrons, which can be used to catalyze CO2 photoreduction to CO under visible light irradiation. In 2019, Lan’s group [64] reported an efficient CO2-to-CH4 conversion, which was achieved in aqueous solution using two crystalline heterogeneous catalysts, NENU-605 and NENU-606. NENU-605 and NENU-606 have a similar host-POM structure constructed by strong reductive {P4MoV6} units and homo/hetero transition metal ions (MnII/CoIIMnII). Notably, the {P4MoV6} cluster including six MoV atoms can serve as a multielectron donor during photocatalysis, while the transition metal ions can function as active sites for adsorbing and activating CO2 molecules. Additionally, the presence of alkali metal ions can assist CO2 capture for photocatalytic reactions. The synergistic combination of the abovementioned components in NENU-605 and NENU-606 effectively facilitates eight-electron transfer for CH4 evolution. Furthermore, NENU-606 containing heterometallic active sites exhibited a higher CH4 selectivity (85.5%) than NENU-605 (76.6%). Subsequently, Lan et al. [65] reported two Keggin-type polytitanates, PTi16 and PTi12, with PO43− as the heteroatom, which display high selectivity and activity for CO2-to-HCOOH photoconversion (Fig. 3).

Reproduced with permission from Ref. [65]. Copyright © 2019, Wiley–VCH
CO2 photoreduction over PTi16 and PTi12.
Su et al. [66] constructed two POM-based hybrids, [Co2.67(SiW12O40)(H2O)4(Htrz)4] (Htrz = 1, 2, 4-triazole) and [Co3(SiW12O40)(H2O)3(Htrz)6Cl], with multinuclear cobalt clusters and a Keggin-type POM under hydrothermal conditions. The photoreduction of CO2 under visible light by these two cobalt-based POMs was investigated using [Ru(bpy)3]2+ as the photosensitizer. Introducing multinuclear Co clusters in these POMs can effectively improve their photocatalytic activity to provide valuable insight into designing high-performance and low-cost molecular catalysts for CO2 photoreduction. Recently, Xu et al. [67] constructed two hourglass-type molybdophosphate hybrids, [Cd(H2O)2DABT]4[Cd(H7P4Mo6O31)2] and (C2H5OH)(C3-H5N2)6[Co3(H6P4Mo6O31)2] (DABT = 3, 3′–diamino–5, 5′–bis(1H–1, 2, 4–triazole)), via a hydrothermal method, and (C2H5OH)(C3H5N2)6[Co3(H6P4Mo6O31)2] can drive CO2 photoreduction with [Ru(bpy)3]2+ as the photosensitizer under visible light irradiation.
Moreover, various strategies were explored to construct cluster-based composite photocatalysts for CO2RR. Recently, Su’s group [68] used a polyoxotitanium cluster [Ti17O24(OPri)20] as a titanium source to anchor ultrafine TiO2–x nanoparticles on ultrathin carbon layers (C-TiO2–x), which were loaded on the g-C3N4 matrix to construct the composite catalyst C-TiO2–x@g-C3N4. The reported C-TiO2–x@g-C3N4 photocatalyst can efficiently reduce CO2 to CO coupled with water oxidation via a two-electron/two-step pathway, and the CO yield can reach up to 12.30 mmol/g within 60 h of visible light irradiation.
Dolbecq et al. [69] constructed a hybrid POM@MOF system by coimmobilizing a Keggin-type POM [PW12O40]3− and catalytically active unit Cp*Rh(bpydc)Cl2 (bpydc = 2, 2′-bipyridine-5,5′-dicarboxylic acid) into a Zr(IV)-based metal–organic framework UiO-67 (Fig. 4). DFT calculations identified two possible locations of the POM in the octahedral cavities of the MOF, with the Cp*Rh complex pointing toward an empty pore. Photocatalytic experiments revealed that the (PW12, Cp*Rh)@UiO-67 composite can efficiently catalyze CO2 reduction into formate and hydrogen. The formate production was much enhanced compared to that of the POM-free Cp*Rh@UiO-67 catalyst, which demonstrates the obvious influence of the POM on CO2 photoreduction. Lan’s group [70] reported two stable POM-grafted metalloporphyrin coordination frameworks (POMCFs), which are constructed with reductive Zn-ε-Keggin clusters and visible light-responsive tetrakis(4-carboxylphenyl) porphyrin (H2TCPP) linkers (Fig. 5). Theoretical calculations revealed that the photogenerated carriers of the valence and conduction bands are mostly distributed in the TCPP group and Zn-ε-Keggin cluster, respectively. The composite catalyst exhibits high selectivity for CO2 photoreduction to CH4 (> 96%). Yao et al. [17] isolated a high-nuclear spin Co–V–O cluster stabilized by lacunary Keggin-type POMs, and the resulting [{Co4(O–H)3(VO4)}4(SiW9O34)4]32− polyoxoanion was composed of a {Co4(OH)3(VO4)}4 core stabilized by four lacunary A-α-{SiW9O34} units. A photocatalytic study revealed that this compound can drive CO2-to-CO conversion with high selectivity under visible light irradiation.

Reproduced with permission from Ref. [69]. Copyright © 2020, American Chemical Society
Synthesis of (PW12, Cp*Rh)@UiO-67 for CO2 photoreduction.

Reproduced with permission from Ref. [70]. Copyright © 2018, Nature Publishing Group
a Four Zn atoms tetrahedron-capped Zn-ε-Keggin cluster, b four carboxyl groups of every Zn-TCPP ligand concurrently in contact with four different Zn-ε-Keggin clusters from four POM chains, c proposed mechanism for CO2 photoreduction over POMCFs under visible light irradiation.
Metal-Oxo Cluster-Based Photocatalysts for Energy Conversion
Low-Cost Transition Metal Clusters for Photocatalysis
Recently, increasing attention has been paid to high-nuclear spin metal clusters because of their structural aesthetics [71,72,73], intriguing properties, and potential technological applications. These metal-oxo clusters are widely used in photocatalysis for water splitting and CO2 reduction because of their unusual chemical activity and catalytic features [74]. Artificial photosynthesis is an effective method for achieving sustainable development by directly converting solar energy into storable chemical fuels [75]. Thus far, the directed development of efficient WOCs is still a great challenge in synthetic and analytic chemistry [76]. The cubane-like {CaMn4O5} oxygen evolution active center in PSII has provided an excellent model for designing WOCs, but its structure and mechanism are still under investigation [77, 78]. Cobalt-based clusters are considered economical and robust WOCs and have attracted wide attention in the past decades. In 2015, Patzke’s group [79] reported a series of [CoII3Ln(hmp)4(OAc)5H2O]({CoII3Ln(O-R)4} {CoII3Ln(OR)4} (Ln = Ho–Yb, hmp = 2 – (hydroxymethyl)pyridine) cubane-like WOCs. The {CoII3Ln(OR)4} cubanes facilitated the design and synthesis of a biologically active WOC by combining Ln3+ centers as a substitute for the redox-inactive Ca2+ to combine the flexible aqua-/acetate ligands (Fig. 6a). Subsequently, they introduced active and stable {CoII4O4} cubane into [CoII4(dpy{OH}O)4(Oac)2(H2O)2](ClO4)2(Co4O4-dpk) as a molecular WOC with a {H2O-Co2(OR)2-OH2} edge-site motif [80]. Moreover, they also presented a series of mixed Co/Ni-cubanes [CoIIxNi4–x(dpy{OH}O)4(Oac)2(H2O)2](ClO4)2{CoxNi4–xO4-dpk} as the WOCs (Fig. 6b) [81]. Recently, Kong and coauthors [82] demonstrated a series of bio-inspired heterometallic cubane clusters LnCo3 (Ln = Nd, Eu, and Ce), which can be considered synthetic analogs of the CaMn4O5 cluster. They creatively anchored the LnCo3 on phosphorus-doped graphitic carbon nitrides (PCNs). The combination of LnCo3 clusters and PCN achieves efficient separation of photogenerated carriers and enables rapid production of H2 and O2. Consequently, the resulting LnCo3@PCN composite catalysts show efficient overall water splitting without any sacrificial reagents.
In 2016, Lu et al. [83] reported a binuclear cobalt cluster that can be used as an efficient homogeneous photocatalyst for visible light-driven CO2 reduction with the assistance of a [Ru(phen)3]2+ photosensitizer. The Co–Co synergistic catalytic effect in this binuclear cluster played an important role in improving its catalytic efficiency, which is supported by experimental and DFT results. Subsequently, they succeeded in obtaining a dual-core CoZn catalyst by replacing CoII with ZnII, which significantly improved the activity of photocatalytic reduction of CO2 under lower light intensity [84]. The auxiliary ZnII site shows a strong binding affinity to OH−, which greatly promotes cleavage of O=C–OH to form C–OH to significantly improve the performance of CO2 photoreduction. Further, Zhang et al. [85] explored a strong sensitizing photosensitizer of [Ru(Phen)2(3-pyrenylPhen)]2+ to replace a [Ru(phen)3]2+ photosensitizer. These strong sensitizing photosensitizers can efficiently sensitize the binuclear cobalt cluster for efficient CO2-to-CO conversion to achieve a turnover number of 66,480.
Since the report of TiO2 for water photolysis, semiconductor materials have attracted extensive attention in this field [5]. In recent years, loading metal nanoparticles on semiconductors has been found to efficiently modulate their electronic structure, thus improving photocatalytic performance [86]. However, the photocatalytic mechanism with multidispersed nanoparticles is difficult to understand at the atomic level. Metal-oxo clusters with clear crystal structures are ideal models for understanding the structure–activity relationship. Kong et al. [87] loaded the 3d–4f metal cluster of Ln52Ni56 (Ln = Eu, Gd, Pr, Nd) on the surface of the CdS semiconductor to effectively improve the separation efficiency of photogenerated electrons and holes, thus improving the performance of photocatalytic water splitting. Some of the Ni2+ in the Ln52Ni56 cluster could be replaced by Cd2+ during the cluster loading to form the Eu52Ni56–xCdx/CdS composite catalyst. Among these catalysts, multichannel electron transfer can confer higher photocatalytic performance to Eu52Ni56 than other rare earth homologs, reaching a H2 yield of 33,533 μmol/(h g).
Metal-Oxo Clusters in MOFs for Photocatalysis
Metal–organic framework materials are constructed from various metal cations and organic ligands, and they have rapidly grown into a well-known class of crystalline materials with porous structures [88]. These clusters can be used as connecting nodes to construct MOFs, which can not only exert the activity of clusters but also coordinate with photoactive ligands to improve their photocatalytic efficiency [89]. Kong et al. [90] demonstrated a stable metal–organic framework featuring dinuclear Eu(III)2 clusters as connecting nodes and Ru(phen)3-derived ligands as linkers, which can be used for visible light-driven CO2 reduction (Fig. 7a). Photoexcitation of the metalloligands initiates electron injection into the dinuclear {Eu(II)}2 active sites to selectively reduce CO2 to formate via a two-electron process. The electron transfer from Ru metalloligands to Eu(III)2 catalytic centers is studied via transient absorption and theoretical calculations to reveal the photocatalytic mechanism.

Reproduced with permission from Ref. [90]. Copyright © 2018, Nature Publishing Group, b function of the heterometallic cluster-based organic framework for photocatalysis. Reproduced with permission from Ref. [91]. Copyright © 2020, Wiley–VCH
a Schematic of the light-induced dynamics in Eu-Ru(phen)3-MOF.
In 2020, Lan et al. [91] synthesized a series of stable heterometallic Fe2M cluster-based MOFs, which were used as efficient photocatalysts to achieve artificial photosynthesis for coupling CO2 reduction with H2O oxidation in the absence of an additional sacrificial agent. During visible light excitation, low-valence metal centers can accept electrons to reduce CO2, and the high-valence Fe center can be used for water oxidation (Fig. 7b).
A new strategy was explored by preincorporating metal precursors in the cavity of MOFs followed by in situ reduction to load nanoclusters into the MOF matrix [92, 93]. In 2020, Lin’s group [94] used low-intensity light to generate CuI species in the cavities of a MOF to in situ activate a CuII(HxPO4)y@Ru-UiO catalyst for selective CO2 hydrogenation to EtOH. Recently, Zhang et al. [95] synthesized a series of composite catalysts by integrating a Ru(bpy)3 photosensitizer and single-metal catalysts of [bpy-CuCl2] into a Eu-MOF platform. The copper catalytic active center and the assembly of the Ru(bpy)3 photosensitizer play an important role in significantly improving their photocatalytic performance. The formate yield is up to 3040 μmol/g in 10 h with a selectivity of 99.7%. Systematic studies first revealed that the catalytic process is controlled by the Cu/X synergy to in-situ generate H-bonding between X and the CO2 reduction intermediate in the photocatalytic process (Fig. 8). The selectivity control of HCOO− versus CO can be simply conducted by changing the coordination ligand to the Cu center. Subsequently, cobalt single-site and ultrafine CuPd nanocluster catalysts were integrated into a porphyrin-based MOF to construct the composite photocatalyst (Cu1Pd2)z@PCN-222(Co) (z = 1.3, 2.0, and 3.0 nm) [96]. Upon visible light irradiation, excited porphyrin can concurrently transfer electrons to Co single sites and CuPd nanoclusters, providing the possibility for coupling CO2 photoreduction and Suzuki/Sonogashira reactions. Systematic investigations demonstrate that the close interaction of the CO2 reduction center and carbonylation Suzuki coupling catalyst can promote the direct transfer of CO and CO* between these two catalytic active centers. The collaboration among different components in these composite catalysts highlights a new insight into developing a sustainable protocol for carbonylation reactions using the greenhouse gas CO2 as a C1 source (Fig. 9).

Reproduced with permission from Ref. [95]. Copyright © 2021, American Chemical Society
a Integration of a Ru(bpy)3 photosensitizer and a single-metal catalyst of [bpy-CuCl2] into a Eu-MOF platform for selectivity control of CO2 photoreduction to HCOO– versus CO, b comparison of production yield under different coordination conditions.

Reproduced with permission from Ref. [96]. Copyright © 2021, American Chemical Society
(Cu1Pd2)1.3@PCN-222(Co) photocatalyst for the solar-driven carbonylation Suzuki coupling reaction under CO2 with multicomponent synergy.
Conclusion
Currently, concern is growing that sustainable and clean energy should become an essential energy source for human life. In recent years, researchers have successfully investigated on various cluster photocatalysts for solar energy conversion. POMs and other metal-oxo clusters have exhibited excellent catalytic performance. These multimetal clusters have shown great potential in the field of photocatalysis because of their adjustable components, diverse structures, and multimetal synergistic catalysis with excellent catalytic properties. Some of them have been used as building units to assemble photosensitizers with excellent catalytic performance, which represents a popular research field. These cluster-based photocatalysts with well-defined structures have supplied typical models to show how to construct effective catalysts. However, the current metal clusters are mainly based on homogeneous catalysis with insufficient reusability and require additional photosensitizers and/or sacrificial agents to maintain the catalytic reaction cycle. The large crystal size of POM-based compounds needs to be ground to increase the specific surface area, and the limited light absorption ability and limited number of exposed active sites still need to be greatly improved. Although obstacles still need to be overcome, increasingly more studies have shown the advantages of POMs in the field of photocatalysis, and more effort is needed to explore efficient and robust cluster photocatalysts.
References
Sakakura T, Choi JC, Yasuda H (2007) Transformation of carbon dioxide. Chem Rev 107(6):2365–2387
Marshall J (2014) Solar energy: springtime for the artificial leaf. Nature 510(7503):22–24
Chang XX, Wang T, Gong JL (2016) CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ Sci 9(7):2177–2196
Berardi S, Drouet S, Francàs L et al (2014) Molecular artificial photosynthesis. Chem Soc Rev 43(22):7501–7519
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358):37–38
Dong BB, Cui JY, Gao YY et al (2019) Heterostructure of 1D Ta3N5 nanorod/BaTaO2N nanoparticle fabricated by a one-step ammonia thermal route for remarkably promoted solar hydrogen production. Adv Mater Deerfield Beach Fla 31(15):e1808185
Liu SH, Yang CS, Zha SJ et al (2022) Moderate surface segregation promotes selective ethanol production in CO2 hydrogenation reaction over CoCu catalysts. Angew Chem Int Ed 61(2):e202109027
Guo Q, Liang F, Li XB et al (2019) Efficient and selective CO2 reduction integrated with organic synthesis by solar energy. Chem 5(10):2605–2616
Cheng DF, Zhao ZJ, Zhang G et al (2021) The nature of active sites for carbon dioxide electroreduction over oxide-derived copper catalysts. Nat Commun 12:395
Bian J, Feng JN, Zhang ZQ et al (2019) Dimension-matched zinc phthalocyanine/BiVO4 ultrathin nanocomposites for CO2 reduction as efficient wide-visible-light-driven photocatalysts via a cascade charge transfer. Angew Chem Int Ed 58(32):10873–10878
Huang YM, Du PY, Shi WX et al (2021) Filling COFs with bimetallic nanoclusters for CO2-to-alcohols conversion with H2O oxidation. Appl Catal B Environ 288:120001
Tee SY, Win KY, Teo WS et al (2017) Recent progress in energy-driven water splitting. Adv Sci 4(5):1600337
Kim D, Sakimoto KK, Hong DC et al (2015) Artificial photosynthesis for sustainable fuel and chemical production. Angew Chem Int Ed 54(11):3259–3266
Pan YX, You Y, Xin S et al (2017) Photocatalytic CO2 reduction by carbon-coated indium-oxide nanobelts. J Am Chem Soc 139(11):4123–4129
He HB, Wang G, Chai SC et al (2021) Self-assembled lamellar nanochannels in polyoxometalate-polymer nanocomposites for proton conduction. Chin Chem Lett 32(6):2013–2016
Liu JC, Han Q, Chen LJ et al (2018) Aggregation of giant cerium-bismuth tungstate clusters into a 3D porous framework with high proton conductivity. Angew Chem Int Ed 57(28):8416–8420
Qiao LZ, Song M, Geng AF et al (2019) Polyoxometalate-based high-nuclear cobalt-vanadium-oxo cluster as efficient catalyst for visible light-driven CO2 reduction. Chin Chem Lett 30(6):1273–1276
Chen R, Yan ZH, Kong XJ (2020) Recent advances in first-row transition metal clusters for photocatalytic water splitting. ChemPhotoChem 4(3):157–167
Pan ZH, Weng ZZ, Kong XJ et al (2022) Lanthanide-containing clusters for catalytic water splitting and CO2 conversion. Coord Chem Rev 457:214419
Papatriantafyllopoulou C, Moushi EE, Christou G et al (2016) Filling the gap between the quantum and classical worlds of nanoscale magnetism: giant molecular aggregates based on paramagnetic 3d metal ions. Chem Soc Rev 45(6):1597–1628
Wu TX, Tao Y, He QJ et al (2021) Constructing an unprecedented MnII38 matryoshka doll with a [Mn18(CO3)9] inorganic core and magnetocaloric effect. Chem Commun 57(22):2732–2735
Hu S, Shaner MR, Beardslee JA et al (2014) Amorphous TiO2 coatings stabilize Si, GaAs, and GaP photoanodes for efficient water oxidation. Science 344(6187):1005–1009
Chen CF, Wu AP, Yan HJ et al (2018) Trapping [PMo12O40]3− clusters into pre-synthesized ZIF-67 toward MoxCoxC particles confined in uniform carbon polyhedrons for efficient overall water splitting. Chem Sci 9(21):4746–4755
Zhang ZM, Zhang T, Wang C et al (2015) Photosensitizing metal-organic framework enabling visible-light-driven proton reduction by a wells-Dawson-type polyoxometalate. J Am Chem Soc 137(9):3197–3200
Chen XB, Shen SH, Guo LJ et al (2010) Semiconductor-based photocatalytic hydrogen generation. Chem Rev 110(11):6503–6570
Zou Z, Ye J, Sayama K et al (2001) Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 414(6864):625–627
Kudo A, Miseki Y (2009) Heterogeneous photocatalyst materials for water splitting. Chem Soc Rev 38(1):253–278
Zhang ZY, Lin QP, Kurunthu D et al (2011) Synthesis and photocatalytic properties of a new heteropolyoxoniobate compound: K10[Nb2O2(H2O)2][SiNb12O40]·12H2O. J Am Chem Soc 133(18):6934–6937
Huang P, Qin C, Su ZM et al (2012) Self-assembly and photocatalytic properties of polyoxoniobates: Nb24O72}, {Nb32O96}, and {K12Nb96O288 clusters. J Am Chem Soc 134(34):14004–14010
Li SJ, Liu SM, Liu SX et al (2012) Ta12}/{Ta16 cluster-containing polytantalotungstates with remarkable photocatalytic H2 evolution activity. J Am Chem Soc 134(48):19716–19721
Lv HJ, Guo WW, Wu KF et al (2014) A noble-metal-free, tetra-nickel polyoxotungstate catalyst for efficient photocatalytic hydrogen evolution. J Am Chem Soc 136(40):14015–14018
Han XB, Qin C, Wang XL et al (2017) Bio-inspired assembly of cubane-adjustable polyoxometalate-based high-nuclear nickel clusters for visible light-driven hydrogen evolution. Appl Catal B Environ 211:349–356
Li HL, Zhang M, Lian C et al (2021) Ring-shaped polyoxometalate built by Mn4PW9 and PO4 units for efficient visible-light-driven hydrogen evolution. CCS Chem 3(8):2095–2103
Cui TT, Qin L, Fu FY et al (2021) Pentadecanuclear Fe-containing polyoxometalate catalyst for visible-light-driven generation of hydrogen. Inorg Chem 60(6):4124–4132
Huang P, Wu HY, Huang M et al (2017) A novel Ta/W mixed-addendum polyoxometalate with photocatalytic properties. Dalton Trans 46(31):10177–10180
Jiao YQ, Qin C, Wang XL et al (2014) Redox-controlled δ-Dawson Mn2IIIW17 polyoxometalate with photocatalytic H2 evolution activity. Chem Commun 50(45):5961–5963
Sun WL, He C, Liu T et al (2019) Synergistic catalysis for light-driven proton reduction using a polyoxometalate-based Cu-Ni heterometallic-organic framework. Chem Commun 55(26):3805–3808
Kong XJ, Lin ZK, Zhang ZM et al (2016) Hierarchical integration of photosensitizing metal-organic frameworks and nickel-containing polyoxometalates for efficient visible-light-driven hydrogen evolution. Angew Chem Int Ed 55(22):6411–6416
Tian J, Xu ZY, Zhang DW et al (2016) Supramolecular metal-organic frameworks that display high homogeneous and heterogeneous photocatalytic activity for H2 production. Nat Commun 7:11580
Jiao L, Dong YY, Xin X et al (2021) Facile integration of Ni-substituted polyoxometalate catalysts into mesoporous light-responsive metal-organic framework for effective photogeneration of hydrogen. Appl Catal B Environ 291:120091
Zhang TZ, Yao S, Zhang ZM et al (2014) Grafting transition metal-organic fragments onto W/Ta mixed-addendum nanoclusters for broad-spectrum-driven photocatalysis. ChemPlusChem 79(8):1153–1158
Schönweiz S, Rommel SA, Kübel J et al (2016) Covalent photosensitizer-polyoxometalate-catalyst dyads for visible-light-driven hydrogen evolution. Chemistry 22(34):12002–12005
Amthor S, Knoll S, Heiland M et al (2022) A photosensitizer–polyoxometalate dyad that enables the decoupling of light and dark reactions for delayed on-demand solar hydrogen production. Nat Chem 14(3):321–327
Zhang M, Li HJ, Zhang JH et al (2021) Research advances of light-driven hydrogen evolution using polyoxometalate-based catalysts. Chin J Catal 42(6):855–871
Xing XL, Liu RJ, Yu XL et al (2013) Self-assembly of CdS quantum dots with polyoxometalate encapsulated gold nanoparticles: enhanced photocatalytic activities. J Mater Chem A 1(4):1488–1494
Dong YJ, Han Q, Hu QY et al (2021) Carbon quantum dots enriching molecular nickel polyoxometalate over CdS semiconductor for photocatalytic water splitting. Appl Catal B Environ 293:120214
Zhang M, Xin X, Feng YQ et al (2022) Coupling Ni-substituted polyoxometalate catalysts with water-soluble CdSe quantum dots for ultraefficient photogeneration of hydrogen under visible light. Appl Catal B Environ 303:120893
Zheng M, Ding Y, Cao XH et al (2018) Homogeneous and heterogeneous photocatalytic water oxidation by polyoxometalates containing the most earth-abundant transition metal, iron. Appl Catal B Environ 237:1091–1100
Liang XM, Cao XH, Sun WJ et al (2019) Recent progress in visible light driven water oxidation using semiconductors coupled with molecular catalysts. ChemCatChem 11(24):6190–6202
Luan XQ, Du HT, Kong Y et al (2019) A novel FeS-NiS hybrid nanoarray: an efficient and durable electrocatalyst for alkaline water oxidation. Chem Commun 55(51):7335–7338
Yin QS, Tan JM, Besson C et al (2010) A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 328(5976):342–345
Song FY, Ding Y, Ma BC et al (2013) K7[CoIIICoII(H2O)W11O39]: a molecular mixed-valence Keggin polyoxometalate catalyst of high stability and efficiency for visible light-driven water oxidation. Energy Environ Sci 6(4):1170
Han XB, Zhang ZM, Zhang T et al (2014) Polyoxometalate-based cobalt-phosphate molecular catalysts for visible light-driven water oxidation. J Am Chem Soc 136(14):5359–5366
Han XB, Li YG, Zhang ZM et al (2015) Polyoxometalate-based nickel clusters as visible light-driven water oxidation catalysts. J Am Chem Soc 137(16):5486–5493
Bhunia MK, Yamauchi K, Takanabe K (2014) Harvesting solar light with crystalline carbon nitrides for efficient photocatalytic hydrogen evolution. Angew Chem Int Ed 53(41):11001–11005
Zhang ZM, Yao S, Li YG et al (2013) A polyoxometalate-based single-molecule magnet with a mixed-valent MnIV2MnIII6MnII4 core. Chem Commun 49(25):2515–2517
Santoni MP, La Ganga G, Mollica Nardo V et al (2014) The use of a vanadium species as a catalyst in photoinduced water oxidation. J Am Chem Soc 136(23):8189–8192
Lv HJ, Song J, Geletii YV et al (2014) An exceptionally fast homogeneous carbon-free cobalt-based water oxidation catalyst. J Am Chem Soc 136(26):9268–9271
Paille G, Gomez-Mingot M, Roch-Marchal C et al (2018) A fully noble metal-free photosystem based on cobalt-polyoxometalates immobilized in a porphyrinic metal-organic framework for water oxidation. J Am Chem Soc 140(10):3613–3618
Zhang L, Zhao ZJ, Gong JL (2017) Nanostructured materials for heterogeneous electrocatalytic CO2 reduction and their related reaction mechanisms. Angew Chem Int Ed 56(38):11326–11353
Ding ML, Flaig RW, Jiang HL et al (2019) Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem Soc Rev 48(10):2783–2828
Cui GK, Wang JJ, Zhang SJ (2016) Active chemisorption sites in functionalized ionic liquids for carbon capture. Chem Soc Rev 45(15):4307–4339
Ettedgui J, Diskin-Posner Y, Weiner L et al (2011) Photoreduction of carbon dioxide to carbon monoxide with hydrogen catalyzed by a rhenium (I) phenanthroline-polyoxometalate hybrid complex. J Am Chem Soc 133(2):188–190
Xie SL, Liu J, Dong LZ et al (2019) Hetero-metallic active sites coupled with strongly reductive polyoxometalate for selective photocatalytic CO2-to-CH4 conversion in water. Chem Sci 10(1):185–190
Li N, Liu J, Liu JJ et al (2019) Self-assembly of a phosphate-centered polyoxo-titanium cluster: discovery of the heteroatom Keggin family. Angew Chem Int Ed 58:17260–17264
Yao W, Qin C, Xu N et al (2019) Visible-light CO2 photoreduction of polyoxometalate-based hybrids with different cobalt clusters. CrystEngComm 21(42):6423–6431
Du ZY, Chen Z, Kang RK et al (2020) Two 2D layered P4Mo6 clusters with potential bifunctional properties: proton conduction and CO2 photoreduction. Inorg Chem 59(17):12876–12883
Zhou J, Wu H, Sun CY et al (2018) Ultrasmall C-TiO2−x nanoparticle/g-C3N4 composite for CO2 photoreduction with high efficiency and selectivity. J Mater Chem A 6(43):21596–21604
Benseghir Y, Lemarchand A, Duguet M et al (2020) Co-immobilization of a Rh catalyst and a keggin polyoxometalate in the UiO-67 Zr-based metal-organic framework: in depth structural characterization and photocatalytic properties for CO2 reduction. J Am Chem Soc 142(20):9428–9438
Wang YR, Huang Q, He CT et al (2018) Oriented electron transmission in polyoxometalate-metalloporphyrin organic framework for highly selective electroreduction of CO2. Nat Commun 9:4466
Song YT, Peng YW, Yao S et al (2022) Co-POM@MOF-derivatives with trace cobalt content for highly efficient oxygen reduction. Chin Chem Lett 33(2):1047–1050
Lu YZ, Chen W (2012) Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem Soc Rev 41(9):3594–3623
Zhou M, Higaki T, Hu GX et al (2019) Three-orders-of-magnitude variation of carrier lifetimes with crystal phase of gold nanoclusters. Science 364(6437):279–282
Zhang N, Hong LY, Geng AF et al (2018) Extended structural materials constructed from sulfate-centered Preyssler-type polyoxometalate with excellent electrocatalytic property. Chin Chem Lett 29(9):1409–1412
Chen YS, Kamat PV (2014) Glutathione-capped gold nanoclusters as photosensitizers. Visible light-induced hydrogen generation in neutral water. J Am Chem Soc 136(16):6075–6082
Zhang BB, Sun LC (2019) Artificial photosynthesis: opportunities and challenges of molecular catalysts. Chem Soc Rev 48:2216–2264
Kanady JS, Lin PH, Carsch KM et al (2014) Toward models for the full oxygen-evolving complex of photosystem II by ligand coordination to lower the symmetry of the Mn3CaO4 cubane: demonstration that electronic effects facilitate binding of a fifth metal. J Am Chem Soc 136(41):14373–14376
Zhang CX, Chen CH, Dong HX et al (2015) A synthetic Mn4Ca-cluster mimicking the oxygen-evolving center of photosynthesis. Science 348(6235):690–693
Evangelisti F, Moré R, Hodel F et al (2015) 3d–4f CoII3Ln(OR)4 cubanes as bio-inspired water oxidation catalysts. J Am Chem Soc 137(34):11076–11084
Hutchings GS, Zhang Y, Li J et al (2015) In situ formation of cobalt oxide nanocubanes as efficient oxygen evolution catalysts. J Am Chem Soc 137(12):4223–4229
Song FY, Moré R, Schilling M et al (2017) Co4O4 and CoxNi4-xO4 cubane water oxidation catalysts as surface cut-outs of cobalt oxides. J Am Chem Soc 139(40):14198–14208
Chen R, Zhuang GL, Wang ZY et al (2020) Integration of bio-inspired lanthanide-transition metal cluster and P-doped carbon nitride for efficient photocatalytic overall water splitting. Natl Sci Rev 8(9):nwaa234
Ouyang T, Huang HH, Wang JW et al (2017) A dinuclear cobalt cryptate as a homogeneous photocatalyst for highly selective and efficient visible-light driven CO2 reduction to CO in CH3CN/H2O solution. Angew Chem Int Ed 56(3):738–743
Ouyang T, Wang HJ, Huang HH et al (2018) Dinuclear metal synergistic catalysis boosts photochemical CO2-to-CO conversion. Angew Chem Int Ed 57(50):16480–16485
Wang P, Dong R, Guo S et al (2020) Improving photosensitization for photochemical CO2-to-CO conversion. Natl Sci Rev 7(9):1459–1467
Shang L, Tong B, Yu HJ et al (2016) Hydrogen evolution: CdS nanoparticle-decorated Cd nanosheets for efficient visible light-driven photocatalytic hydrogen evolution. Adv Energy Mater 6(3):1501241
Chen R, Yan ZH, Kong XJ et al (2018) Integration of lanthanide-transition-metal clusters onto CdS surfaces for photocatalytic hydrogen evolution. Angew Chem Int Ed 57(51):16796–16800
Zhu QL, Xu Q (2014) Metal-organic framework composites. Chem Soc Rev 43(16):5468–5512
Zhang T, Lin WB (2014) Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem Soc Rev 43(16):5982–5993
Yan ZH, Du MH, Liu JX et al (2018) Photo-generated dinuclear {Eu(II)}2 active sites for selective CO2 reduction in a photosensitizing metal-organic framework. Nat Commun 9:3353
Dong LZ, Zhang L, Liu J et al (2020) Stable heterometallic cluster-based organic framework catalysts for artificial photosynthesis. Angew Chem Int Ed 59(7):2659–2663
Wang C, DeKrafft KE, Lin WB (2012) Pt nanoparticles@photoactive metal-organic frameworks: efficient hydrogen evolution via synergistic photoexcitation and electron injection. J Am Chem Soc 134(17):7211–7214
Horiuchi Y, Toyao T, Saito M et al (2012) Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti(IV) metal-organic framework. J Phys Chem C 116(39):20848–20853
Zeng LZ, Wang ZY, Wang YK et al (2020) Photoactivation of Cu centers in metal-organic frameworks for selective CO2 conversion to ethanol. J Am Chem Soc 142(1):75–79
Zhuo TC, Song Y, Zhuang GL et al (2021) H-bond-mediated selectivity control of formate versus CO during CO2 photoreduction with two cooperative Cu/X sites. J Am Chem Soc 143(16):6114–6122
Fu SS, Yao S, Guo S et al (2021) Feeding carbonylation with CO2 via the synergy of single-site/nanocluster catalysts in a photosensitizing MOF. J Am Chem Soc 143(49):20792–20801
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 21671113), the Science and Technology of Henan province in 2018 (No. 182102310873), 2019 Special Project of Nanyang Normal University (Nos. 2019ZX009 and 2019QN011), Project of Young Backbone Teachers in Colleges and Universities of Henan Province (No. 2020GGJS180) and 2019 Henan Higher Education Teaching Reform Research and Practice Project (No. 2019SJGLX093Y).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lan, Q., Jin, S., Yang, B. et al. Metal-Oxo Cluster Catalysts for Photocatalytic Water Splitting and Carbon Dioxide Reduction. Trans. Tianjin Univ. 28, 214–225 (2022). https://doi.org/10.1007/s12209-022-00324-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-022-00324-z