Abstract
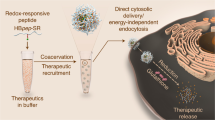
Electrostatic complexation of a cationic MAPKAP kinase 2 inhibitory (MK2i) peptide with the anionic, pH-responsive polymer poly(propylacrylic acid) (PPAA) yields MK2i nano-polyplexes (MK2i-NPs) that significantly increase peptide uptake and intracellular retention. This study focused on elucidating the mechanism of MK2i-NP cellular uptake and intracellular trafficking in vascular smooth muscle cells. Small molecule inhibition of various endocytic pathways showed that MK2i-NP cellular uptake involves both macropinocytosis and clathrin mediated endocytosis, whereas the free peptide exclusively utilizes clathrin mediated endocytosis for cell entry. Scanning electron microscopy studies revealed that MK2i-NPs, but not free MK2i peptide, induce cellular membrane ruffling consistent with macropinocytosis. TEM confirmed that MK2i-NPs induce macropinosome formation and achieve MK2i endo-lysosomal escape and cytosolic delivery. Finally, a novel technique based on recruitment of Galectin-8-YFP was utilized to demonstrate that MK2i-NPs cause endosomal disruption within 30 min of uptake. These new insights on the relationship between NP physicochemical properties and cellular uptake and trafficking can potentially be applied to further optimize the MK2i-NP system and more broadly toward the rational engineering of nano-scale constructs for the intracellular delivery of biologic drugs.






Similar content being viewed by others
References
Barondes, S. H., D. N. Cooper, M. A. Gitt, and H. Leffler. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 269:20807–20810, 1994.
Behr, J. P. The proton sponge: a trick to enter cells the viruses did not exploit. CHIMIA Int. J. Chem. 51:34–36, 1997.
Berguig, G. Y., et al. Intracellular delivery and trafficking dynamics of a lymphoma-targeting antibody-polymer conjugate. Mol. Pharm. 9:3506–3514, 2012.
Bernard, A., and D. J. Klionsky. Toward an understanding of autophagosome-lysosome fusion: the unsuspected role of ATG14. Autophagy 11:583–584, 2015.
Bickel, P. E., and M. W. Freeman. Rabbit aortic smooth muscle cells express inducible macrophage scavenger receptor messenger RNA that is absent from endothelial cells. J. Clin. Invest 90:1450–1457, 1992.
Borsello, T., et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med 9:1180–1186, 2003.
Boussif, O., et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 92:7297–7301, 1995.
Boyle, K. B., and F. Randow. The role of “eat-me” signals and autophagy cargo receptors in innate immunity. Curr. Opin. Microbiol. 16:339–348, 2013.
Brown, M. S., S. K. Basu, J. R. Falck, Y. K. Ho, and J. L. Goldstein. The scavenger cell pathway for lipoprotein degradation: Specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J. Supramol. Struct. 13:67–81, 1980.
Brugnano, J., J. McMasters, and A. Panitch. Characterization of endocytic uptake of MK2-inhibitor peptides. J. Pept. Sci. 19:629–638, 2013.
Canton, J., D. Neculai, and S. Grinstein. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 13:621–634, 2013.
Convertine, A. J., D. S. W. Benoit, C. L. Duvall, A. S. Hoffman, and P. S. Stayton. Development of a novel endosomolytic diblock copolymer for siRNA delivery. J. Control. Release 133:221–229, 2009.
Convertine, A. J., D. S. W. Benoit, C. L. Duvall, A. S. Hoffman, and P. S. Stayton. Development of a novel endosomolytic diblock copolymer for siRNA delivery. J. Control. Release 133:221–229, 2009.
Copolovici, D. M., K. Langel, E. Eriste, and Ü. Langel. Cell-penetrating peptides: design, synthesis, and applications. ACS Nano. 8:1972–1994, 2014.
De Smedt, S. C., J. Demeester, and W. E. Hennink. Cationic polymer based gene delivery systems. Pharm. Res. 17:113–126, 2000.
Deshayes, S., M. C. Morris, G. Divita, and F. Heitz. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell. Mol. Life Sci 62:1839–1849, 2005.
Duvall, C. L., A. J. Convertine, D. S. W. Benoit, A. S. Hoffman, and P. S. Stayton. Intracellular delivery of a proapoptotic peptide via conjugation to a RAFT synthesized endosomolytic polymer. Mol. Pharm. 7:468–476, 2010.
Evans, B. C., K. M. Hocking, K. V. Kilchrist, E. S. Wise, C. M. Brophy, and C. L. Duvall. Endosomolytic nano-polyplex platform technology for cytosolic peptide delivery to inhibit pathological vasoconstriction. ACS Nano. 9:5893–5907, 2015.
Evans, B. C., et al. MK2 inhibitory peptide delivered in nanopolyplexes prevents vascular graft intimal hyperplasia. Sci Transl Med 7:291ra95, 2015.
Falcone, S., E. Cocucci, P. Podini, T. Kirchhausen, E. Clementi, and J. Meldolesi. Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events. J. Cell Sci. 119:4758–4769, 2006.
Felgner, J. H., et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem. 269:2550–2561, 1994.
Flynn, C. R., et al. Internalization and intracellular trafficking of a PTD-conjugated anti-fibrotic peptide, AZX100, in human dermal keloid fibroblasts. J. Pharm. Sci. 99:3100–3121, 2010.
Furuta, N., and A. Amano. SNARE mediates autophagosome–lysosome fusion. J. Oral Biosci. 54:83–85, 2012.
Gump, J. M., and S. F. Dowdy. TAT transduction: the molecular mechanism and therapeutic prospects. Trends Mol. Med. 13:443–448, 2007.
Hayess, K., and R. Benndorf. Effect of protein kinase inhibitors on activity of mammalian small heat-shock protein (HSP25) kinase. Biochem. Pharmacol. 53:1239–1247, 1997.
Hughes, L. D., R. J. Rawle, and S. G. Boxer. Choose your label wisely: water-soluble fluorophores often interact with lipid bilayers. PLoS One 9:e87649, 2014.
Kaplan, I. M., J. S. Wadia, and S. F. Dowdy. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J. Control. Release 102:247–253, 2005.
Khalil, I. A., K. Kogure, H. Akita, and H. Harashima. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 58:32–45, 2006.
Knighton, D. R., J. H. Zheng, L. F. Ten Eyck, N. H. Xuong, S. S. Taylor, and J. M. Sowadski. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253:414–420, 1991.
Kotlyarov, A., et al. Distinct cellular functions of MK2. Mol. Cell. Biol. 22:4827–4835, 2002.
Li, H., M. Freeman, and P. Libby. Regulation of smooth muscle cell scavenger receptor expression in vivo by atherogenic diets and in vitro by cytokines. J. Clin. Invest. 1995. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC295387/
Lopes, L. B., C. Flynn, P. Komalavilas, A. Panitch, C. M. Brophy, and B. L. Seal. Inhibition of HSP27 phosphorylation by a cell-permeant MAPKAP Kinase 2 inhibitor. Biochem. Biophys. Res. Commun. 382:535–539, 2009.
Lopes, L. B., et al. Cell permeant peptide analogues of the small heat shock protein, HSP20, reduce TGF-β1-induced CTGF expression in keloid fibroblasts. J. Invest. Dermatol. 129:590–598, 2008. http://www.nature.com/doifinder/10.1038/jid.2008.264
Meng, W., et al. Structure of mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 suggests a bifunctional switch that couples kinase activation with nuclear export. J. Biol. Chem. 277:37401–37405, 2002.
Mercer, J., and A. Helenius. Virus entry by macropinocytosis. Nat. Cell Biol. 11:510–520, 2009.
Nakase, I., N. B. Kobayashi, T. Takatani-Nakase, and T. Yoshida. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci. Rep. 5:10300, 2015.
Richard, J. P., et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 278:585–590, 2003.
Shibutani, S. T., and T. Yoshimori. Autophagosome formation in response to intracellular bacterial invasion. Cell. Microbiol. 16:1619–1626, 2014.
Sorkin, A., and M. von Zastrow. Signal transduction and endocytosis: close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 3:600–614, 2002.
Stewart, S. A., et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9:493–501, 2003.
Thurston, T. L. M., M. P. Wandel, N. von Muhlinen, A. Foeglein, and F. Randow. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482:414–418, 2012.
Varkouhi, A. K., M. Scholte, G. Storm, and H. J. Haisma. Endosomal escape pathways for delivery of biologicals. J. Control. Release 151:220–228, 2011.
Verdine, G. L., and G. J. Hilinski. Stapled peptides for intracellular drug targets. Methods Enzymol. 503:3–33, 2012.
Wittrup, A., et al. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 33:870–876, 2015.
Acknowledgments
We thank Dr. Felix Randow and Dr. Bob Weinburg for kind gifts of plasmids via AddGene.com. We thank Dr. Janice Williams for imaging support and electron microscopy expertise. Confocal imaging, transmission electron microscopy, and scanning electron microscopy were performed in part through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource (supported by NIH Grants CA68485, DK20593, DK58404, DK59637 and EY08126). Dynamic light scattering was conducted at the Vanderbilt Institute of Nanoscale Sciences and Engineering. This work was supported by the American Heart Association (11SDG4890030), National Institutes of Health/National Heart, Lung, and Blood Institute (R21 HL110056 and R01 HL122347), and a National Science Foundation Graduate Research Fellowship to K.V.K. (0909667 and 1445197).
Conflict of Interest
KVK, BCE, CMB, and CLD report grant support from the National Institutes of Health and the American Heart Association; KVK additionally reports grant support from National Science Foundation Graduate Research Fellowship Program. During the conduct of the study, authors disclose non-financial support from Moerae Matrix, Inc., outside the submitted work. CMB is chief scientific officer and a shareholder of Moerae Matrix, Inc. BCE, CMB, and CLD are inventors listed on patent PCT/US2014/033873, licensed by Moerae Matrix, Inc. MK2i is known commercially known as MMI-0100 and is being developed by Moerae Matrix, Inc. for clinical use (ClinicalTrials.gov Identifier: NCT02515396).
Ethical Standards
No human or animal studies were carried out by the authors for the completion of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Tejal Desai oversaw the review of this article.
Craig L. Duvall is an Associate Professor of Biomedical Engineering (BME) at Vanderbilt University. He completed his undergraduate studies at the University of Kentucky in 2001 and immediately started his doctoral studies in BME at Georgia Tech and Emory University. Robert Guldberg, a Mechanical/Biomedical Engineer from Georgia Tech, and W. Robert Taylor, a cardiologist from Emory, jointly directed Dr. Duvall’s Ph.D. work. In 2007, Dr. Duvall joined the Bioengineering laboratories of Patrick Stayton and Allan Hoffman at the University of Washington developing polymeric drug delivery technologies as a postdoctoral researcher. Based on the foundations built from these combined experiences, the Duvall Advanced Therapeutics Laboratory (ATL) was launched at Vanderbilt in 2010. The ATL is funded by grants from NIH, DOD, NSF, AHA, and ADA and focuses on the development of novel drug delivery technologies for applications in regenerative medicine and breast cancer therapy.

This article is part of the 2016 Young Innovators Issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 27623 kb)
Rights and permissions
About this article
Cite this article
Kilchrist, K.V., Evans, B.C., Brophy, C.M. et al. Mechanism of Enhanced Cellular Uptake and Cytosolic Retention of MK2 Inhibitory Peptide Nano-polyplexes. Cel. Mol. Bioeng. 9, 368–381 (2016). https://doi.org/10.1007/s12195-016-0446-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-016-0446-7