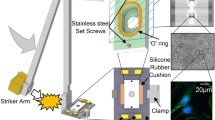
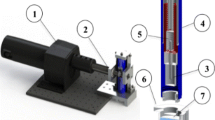
Abstract
Concussive injuries are a subset of traumatic brain injuries (TBIs) during which impact of the head against another object leads to rapid deceleration and deformation of brain tissue. Mechanically triggered changes in central nervous system (CNS) cell behavior following this type of injury are believed to play a role in the pathological response to TBI observed clinically. To study the mechanobiology of CNS cells under controlled conditions an in vitro impact tester was developed to mimic the impact deceleration and strain parameters associated with concussive injuries. Similar in concept to a automotive crash test, the bench top system was capable of delivering decelerations from 0 to 300g and biaxial strains from 0 to 25% over durations from 1 to 20 ms to pure populations of cells grown in both 2d and 3d culture. The small footprint (1′ × 1′) and inexpensive design, utilizing many off the shelf components, makes it a useful lab based system. Utilizing the system, neuronally differentiated PC12 cells and cortical astrocytes were treated with both low (50 g + 10% stain) and high (100 g + 20%) single impact conditioning. Following both low and high impact testing, significant PC12 cell detachment was measured, suggesting as others have shown, a susceptibility of neurons to impact conditioning. Alternatively, astrocyte cultures showed no evidence of cellular detachment in response to either low or high impact conditioning, but significant reductions to the production of TGFβ1 and MCP-1 were measured following high impact conditioning. The relationship between impact and the production of key wound healing cytokines by astrocytes not only supports astrocyte mechano-sensitivity, but also suggests their involvement in the wound healing response following TBI. We believe this device provides a unique toolset that will aid in the exploration of cellular impact mechanobiology and that ultimately an improved understanding of cell mechanobiology will help guide the development of diagnostic techniques and therapeutic interventions targeting cells with known roles in the response to TBI.







Similar content being viewed by others
References
Bain, A. C., and D. F. Meaney. Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J. Biomech. Eng. 122:615–622, 2000.
Bayly, P. V., E. E. Black, R. C. Pedersen, E. P. Leister, and G. M. Genin. In vivo imaging of rapid deformation and strain in an animal model of traumatic brain injury. J. Biomech. 39:1086–1095, 2006.
Bayly, P. V., T. S. Cohen, E. P. Leister, D. Ajo, E. C. Leuthardt, and G. M. Genin. Deformation of the human brain induced by mild acceleration. J. Neurotrauma 22:845–856, 2005.
Bayly, P. V., S. Ji, S. K. Song, R. J. Okamoto, P. Massouros, and G. M. Genin. Measurement of strain in physical models of brain injury: a method based on HARP analysis of tagged magnetic resonance images (MRI). J. Biomech. Eng. 126:523–528, 2004.
Cater, H. L., D. Gitterman, S. M. Davis, C. D. Benham, B. Morrison, 3rd, and L. E. Sundstrom. Stretch-induced injury in organotypic hippocampal slice cultures reproduces in vivo post-traumatic neurodegeneration: role of glutamate receptors and voltage-dependent calcium channels. J. Neurochem. 101:434–447, 2007.
Cobb, B. R., J. E. Urban, E. M. Davenport, S. Rowson, S. M. Duma, J. A. Maldjian, C. T. Whitlow, A. K. Powers, and J. D. Stitzel. Head impact exposure in youth football: elementary school ages 9–12 years and the effect of practice structure. Ann. Biomed. Eng. 41:2463–2473, 2013.
Coronado, V. G., L. Xu, S. V. Basavaraju, L. C. McGuire, M. M. Wald, M. D. Faul, B. R. Guzman, and J. D. Hemphill. Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveill Summ. 60:1–32, 2011.
Crisco, J. J., R. Fiore, J. G. Beckwith, J. J. Chu, P. G. Brolinson, S. Duma, T. W. McAllister, A. C. Duhaime, and R. M. Greenwald. Frequency and location of head impact exposures in individual collegiate football players. J. Athl. Train. 45:549–559, 2010.
Crisco, J. J., B. J. Wilcox, J. G. Beckwith, J. J. Chu, A. C. Duhaime, S. Rowson, S. M. Duma, A. C. Maerlender, T. W. McAllister, and R. M. Greenwald. Head impact exposure in collegiate football players. J. Biomech. 44:2673–2678, 2011.
Cullen, D. K., C. M. Simon, and M. C. LaPlaca. Strain rate-dependent induction of reactive astrogliosis and cell death in three-dimensional neuronal-astrocytic co-cultures. Brain Res. 1158:103–115, 2007.
Eckner, J. T., M. Sabin, J. S. Kutcher, and S. P. Broglio. No evidence for a cumulative impact effect on concussion injury threshold. J. Neurotrauma 28:2079–2090, 2011.
Freed, L. E., and G. Vunjak-Novakovic. Spaceflight bioreactor studies of cells and tissues. Adv. Space Biol. Med. 8:177–195, 2002.
Geddes-Klein, D. M., K. B. Schiffman, and D. F. Meaney. Mechanisms and consequences of neuronal stretch injury in vitro differ with the model of trauma. J. Neurotrauma 23:193–204, 2006.
Gharaee-Kermani, M., E. M. Denholm, and S. H. Phan. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J. Biol. Chem. 271:17779–17784, 1996.
Greene, L. A., and A. S. Tischler. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 73:2424–2428, 1976.
Ho, L., W. Zhao, K. Dams-O’Connor, C. Y. Tang, W. Gordon, E. R. Peskind, S. Yemul, V. Haroutunian, and G. M. Pasinetti. Elevated plasma MCP-1 concentration following traumatic brain injury as a potential “predisposition” factor associated with an increased risk for subsequent development of Alzheimer’s disease. J. Alzheimers Dis. 31:301–313, 2012.
Israelsson, C., Y. Wang, A. Kylberg, C. G. Pick, B. J. Hoffer, and T. Ebendal. Closed head injury in a mouse model results in molecular changes indicating inflammatory responses. J. Neurotrauma 26:1307–1314, 2009.
King, A. I. Fundamentals of impact biomechanics: Part I-Biomechanics of the head, neck, and thorax. Annu. Rev. Biomed. Eng. 2:55–81, 2000.
Lasher, R. A., J. C. Wolchok, M. K. Parikh, J. P. Kennedy, and R. W. Hitchcock. Design and characterization of a modified T-flask bioreactor for continuous monitoring of engineered tissue stiffness. Biotechnol. Prog. 26:857–864, 2010.
Lee, A. A., T. Delhaas, L. K. Waldman, D. A. MacKenna, F. J. Villarreal, and A. D. McCulloch. An equibiaxial strain system for cultured cells. Am. J. Physiol. 271:C1400–C1408, 1996.
McGraw, J., G. W. Hiebert, and J. D. Steeves. Modulating astrogliosis after neurotrauma. J. Neurosci. Res. 63:109–115, 2001.
McKeon, R. J., A. Hoke, and J. Silver. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp. Neurol. 136:32–43, 1995.
McKeon, R. J., M. J. Jurynec, and C. R. Buck. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J. Neurosci. 19:10778–10788, 1999.
Morganti-Kossmann, M. C., V. H. Hans, P. M. Lenzlinger, R. Dubs, E. Ludwig, O. Trentz, and T. Kossmann. TGF-beta is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood–brain barrier function. J. Neurotrauma 16:617–628, 1999.
Mulder, M. M., R. W. Hitchcock, and P. A. Tresco. Skeletal myogenesis on elastomeric substrates: implications for tissue engineering. J. Biomater. Sci. Polym. Ed. 9:731–748, 1998.
Myer, D. J., G. G. Gurkoff, S. M. Lee, D. A. Hovda, and M. V. Sofroniew. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129:2761–2772, 2006.
Polikov, V. S., E. C. Su, M. A. Ball, J. S. Hong, and W. M. Reichert. Control protocol for robust in vitro glial scar formation around microwires: essential roles of bFGF and serum in gliosis. J. Neurosci. Methods 181:170–177, 2009.
Ralay, Ranaivo H., S. M. Zunich, N. Choi, J. N. Hodge, and M. S. Wainwright. Mild stretch-induced injury increases susceptibility to interleukin-1beta-induced release of matrix metalloproteinase-9 from astrocytes. J. Neurotrauma 28:1757–1766, 2011.
Robertson, C. L., M. Saraswati, S. Scafidi, G. Fiskum, P. Casey, and M. C. McKenna. Cerebral glucose metabolism in an immature rat model of pediatric traumatic brain injury. J. Neurotrauma 30(24):2066–2072, 2013.
Rolls, A., R. Shechter, and M. Schwartz. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 10:235–241, 2009.
Rowson, S., G. Brolinson, M. Goforth, D. Dietter, and S. Duma. Linear and angular head acceleration measurements in collegiate football. J. Biomech. Eng. 131:061016, 2009.
Smith, G. M., and C. Strunz. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia 52:209–218, 2005.
Takeuchi, S., H. Nawashiro, S. Sato, S. Kawauchi, K. Nagatani, H. Kobayashi, N. Otani, H. Osada, K. Wada, and K. Shima. A better mild traumatic brain injury model in the rat. Acta Neurochir. Suppl. 118:99–101, 2013.
Urban, J. E., E. M. Davenport, A. J. Golman, J. A. Maldjian, C. T. Whitlow, A. K. Powers, and J. D. Stitzel. Head impact exposure in youth football: high school ages 14 to 18 years and cumulative impact analysis. Ann. Biomed. Eng. 41:2474–2487, 2013.
Vermeij, J. D., H. Aslami, K. Fluiter, J. J. Roelofs, W. M. van den Bergh, N. P. Juffermans, M. J. Schultz, K. van der Sluijs, D. van de Beek, and D. J. van Westerloo. Traumatic brain injury in rats induces lung injury and systemic immune suppression. J. Neurotrauma 30(24):2073–2079, 2013.
Vonder, Haar C., D. M. Friend, D. B. Mudd, and J. S. Smith. Successive bilateral frontal controlled cortical impact injuries show behavioral savings. Behav. Brain Res. 240:153–159, 2013.
Vorselen, D., W. H. Roos, F. C. Mackintosh, G. J. Wuite, and J. J. van Loon. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 28:536–547, 2014.
Wolchok, J. C., C. Brokopp, C. J. Underwood, and P. A. Tresco. The effect of bioreactor induced vibrational stimulation on extracellular matrix production from human derived fibroblasts. Biomaterials 30:327–335, 2009.
Wolchok, J. C., and P. A. Tresco. The isolation of cell derived extracellular matrix constructs using sacrificial open-cell foams. Biomaterials 31(36):9595–9603, 2010.
Wolchok, J. C., and P. A. Tresco. Using vocally inspired mechanical conditioning to enhance the synthesis of a cell-derived biomaterial. Ann. Biomed. Eng. 41:2358–2366, 2013.
Zhang, D., Y. Hu, Q. Sun, J. Zhao, Z. Cong, H. Liu, M. Zhou, K. Li, and C. Hang. Inhibition of transforming growth factor beta-activated kinase 1 confers neuroprotection after traumatic brain injury in rats. Neuroscience 238:209–217, 2013.
Zohar, O., S. Schreiber, V. Getslev, J. P. Schwartz, P. G. Mullins, and C. G. Pick. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience 118:949–955, 2003.
Conflict of Interest
Zach Heller, Joe Wyatt, Anna Arnaud, and Jeff Wolchok declare that they have no conflicts of interest.
Ethical Standards
No human or animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ellen Arruda oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Heller, Z., Wyatt, J., Arnaud, A. et al. An In Vitro Impact Model for the Study of Central Nervous System Cell Mechanobiology. Cel. Mol. Bioeng. 7, 521–531 (2014). https://doi.org/10.1007/s12195-014-0347-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-014-0347-6