Abstract
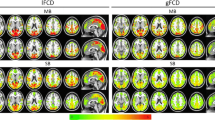
In multisite studies, differences in imaging acquisition systems could affect the reproducibility of the results when examining changes in brain function using resting-state functional magnetic resonance imaging (rs-fMRI). This is also important for longitudinal studies, in which changes in equipment settings can occur. This study examined the reproducibility of functional connectivity (FC) metrics estimated from rs-fMRI data acquired using scanner receiver coils with different numbers of channels. This study involved 80 rs-fMRI datasets from 20 healthy volunteers scanned in two independent imaging sessions using both 12- and 32-channel coils for each session. We used independent component analysis (ICA) to evaluate the FC of canonical resting-state networks (RSNs) and graph theory to calculate several whole-brain network metrics. The effect of global signal regression (GSR) as a preprocessing step was also considered. Comparisons within and between receiver coils were performed. Irrespective of the GSR, RSNs derived from rs-fMRI data acquired using the same receiver coil were reproducible, but not from different receiver coils. However, both the GSR and the channel count of the receiver coil have discernible effects on the reproducibility of network metrics estimated using whole-brain network analysis. The data acquired using the 32-channel coil tended to have better reproducibility than those acquired using the 12-channel coil. Our findings suggest that the reproducibility of FC metrics estimated from rs-fMRI data acquired using different receiver coils showed some level of dependence on the preprocessing method and the type of analysis performed.





Similar content being viewed by others
References
Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. https://doi.org/10.1002/mrm.1910340409.
Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. https://doi.org/10.1002/hbm.460020107.
Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8. https://doi.org/10.1073/pnas.0135058100.
Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc B Biol Sci. 2005;360:1001–13. https://doi.org/10.1098/rstb.2005.1634.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. https://doi.org/10.1523/JNEUROSCI.5587-06.2007.
Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–42. https://doi.org/10.1073/pnas.0308627101.
Dai Z, Yan C, Li K, Wang Z, Wang J, Cao M, et al. Identifying and mapping connectivity patterns of brain network hubs in Alzheimer’s disease. Cereb Cortex. 2015;25:3723–42. https://doi.org/10.1093/cercor/bhu246.
Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15:483–506. https://doi.org/10.1016/j.tics.2011.08.003.
Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. https://doi.org/10.1038/nrn2575.
Chen S, Ross TJ, Zhan W, Myers CS, Chuang KS, Heishman SJ, et al. Group independent component analysis reveals consistent resting-state networks across multiple sessions. Brain Res. 2008;1239:141–51. https://doi.org/10.1016/j.brainres.2008.08.028.
Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–53. https://doi.org/10.1073/pnas.0601417103.
Choe AS, Jones CK, Joel SE, Muschelli J, Belegu V, Caffo BS, et al. Reproducibility and temporal structure in weekly resting-state fMRI over a period of 3.5 years. PLoS ONE. 2015;10:e0140134. https://doi.org/10.1371/journal.pone.0140134.
Bagarinao E, Tsuzuki E, Yoshida Y, Ozawa Y, Kuzuya M, Otani T, et al. Effects of gradient coil noise and gradient coil replacement on the reproducibility of resting state networks. Front Hum Neurosci. 2018;12:148. https://doi.org/10.3389/fnhum.2018.00148.
Huang L, Wang X, Baliki MN, Wang L, Apkarian AV, Parrish TB. Reproducibility of structural, resting-state BOLD and DTI data between identical scanners. PLoS ONE. 2012;7:e47684. https://doi.org/10.1371/journal.pone.0047684.
Paolini M, Keeser D, Ingrisch M, Werner N, Kindermann N, Reiser M, et al. Resting-state networks in healthy adult subjects: a comparison between a 32-element and an 8-element phased array head coil at 3.0 Tesla. Acta radiol. 2015;56:605–13. https://doi.org/10.1177/0284185114567703.
Anteraper SA, Whitfield-Gabrieli S, Keil B, Shannon S, Gabrieli JD, Triantafyllou C. Exploring functional connectivity networks with multichannel brain array coils. Brain Connect. 2013;3:302–15. https://doi.org/10.1089/brain.2012.0113.
Power JD, Plitt M, Laumann TO, Martin A. Sources and implications of whole-brain fMRI signals in humans. Neuroimage. 2017;146:609–25. https://doi.org/10.1016/j.neuroimage.2016.09.038.
Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–73. https://doi.org/10.1016/j.neuroimage.2016.11.052.
Li J, Bolt T, Bzdok D, Nomi JS, Yeo BTT, Spreng RN, et al. Topography and behavioral relevance of the global signal in the human brain. Sci Rep. 2019;9:14286. https://doi.org/10.1038/s41598-019-50750-8.
Yang GJ, Murray JD, Glasser M, Pearlson GD, Krystal JH, Schleifer C, et al. Altered global signal topography in schizophrenia. Cereb Cortex. 2017;27:5156–69. https://doi.org/10.1093/cercor/bhw297.
Mugler JP, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med. 1990;15:152–7. https://doi.org/10.1002/mrm.1910150117.
Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. https://doi.org/10.1016/j.neuroimage.2005.02.018.
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. https://doi.org/10.1016/j.neuroimage.2011.10.018.
Triantafyllou C, Hoge RD, Krueger G, Wiggins CJ, Potthast A, Wiggins GC, et al. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. Neuroimage. 2005;26:243–50. https://doi.org/10.1016/j.neuroimage.2005.01.007.
Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–65. https://doi.org/10.1093/cercor/bhr099.
Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc Natl Acad Sci. 2009;106:7209–14. https://doi.org/10.1073/pnas.0811879106.
Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. https://doi.org/10.1002/hbm.1058.
Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. https://doi.org/10.1016/j.neuroimage.2008.03.061.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. https://doi.org/10.1006/nimg.2001.0978.
Wang J, Wang X, Xia M, Liao X, Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. 2015;9:386. https://doi.org/10.3389/fnhum.2015.00386.
Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70:334–42. https://doi.org/10.1016/j.biopsych.2011.05.018.
He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132:3366–79. https://doi.org/10.1093/brain/awp089.
Li L, Zeng L, Lin Z-J, Cazzell M, Liu H. Tutorial on use of intraclass correlation coefficients for assessing interest reliability and its application in functional near-infrared spectroscopy–based brain imaging. J Biomed Opt. 2015;20: 050801. https://doi.org/10.1117/1.JBO.20.5.050801.
Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56:216–23. https://doi.org/10.1002/mrm.20925.
Albrecht J, Burke M, Haegler K, Schöpf V, Kleemann AM, Paolini M, et al. Potential impact of a 32-channel receiving head coil technology on the results of a functional MRI paradigm. Clin Neuroradiol. 2010;20:223–9. https://doi.org/10.1007/s00062-010-0029-2.
Kaza E, Klose U, Lotze M. Comparison of a 32-channel with a 12-channel head coil: are there relevant improvements for functional imaging? J Magn Reson Imaging. 2011;34:173–83. https://doi.org/10.1002/jmri.22614.
Arnold S, Whitfield-Gabrieli S, Shannon S, Gabrieli J, Triantafyllou C. Improved detection of functional connectivity MRI with 32-channel phased array head coil. Proc Intl Soc Mag Reson Med. 2011;19:1637.
Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;90:449–68. https://doi.org/10.1016/j.neuroimage.2013.11.046.
Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 2014;95:232–47. https://doi.org/10.1016/j.neuroimage.2014.03.034.
Erdoğan SB, Tong Y, Hocke LM, Lindsey KP, de Frederick B. Correcting for blood arrival time in global mean regression enhances functional connectivity analysis of resting state fMRI-BOLD signals. Front Hum Neurosci. 2016;10:311. https://doi.org/10.3389/fnhum.2016.00311.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Ageing and Dementia Control; 26117002) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
Funding
Ministry of Education, Culture, Sports, Science and Technology (MEXT), 26117002, Gen Sobue.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board (IRB) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Human/Animal rights
This article does not contain any studies with animals performed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kato, S., Bagarinao, E., Isoda, H. et al. Reproducibility of functional connectivity metrics estimated from resting-state functional MRI with differences in days, coils, and global signal regression. Radiol Phys Technol 15, 298–310 (2022). https://doi.org/10.1007/s12194-022-00670-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12194-022-00670-6