Abstract
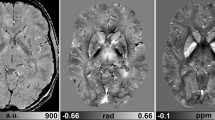
The purpose of our study was to clarify the dependence of quantitative susceptibility mapping (QSM) on echo time (TE). We constructed a phantom consisting of six tubes; three tubes were filled with different concentrations (0.5, 1.0, and 2.5 mM) of gadopentetate dimeglumine (Gd-DTPA), and three were filled with different concentrations (100, 200, and 350 mg/mL) of calcium hydroxyapatite. Real and imaginary images from multi-echo spoiled gradient-echo data (12 echoes) were acquired. We then used four datasets with three serial echoes. The QSM procedure consists of four steps: field map estimation, phase unwrapping, background removal, and dipole inversion. For each sample, we compared the measured mean susceptibility value with the theoretical susceptibility value and conducted a linear regression analysis. Accordingly, the relationship between the measured susceptibility and concentration of Gd-DTPA was shown to agree well with the theoretical values (TEs = 16.4, 20.8, and 25.2 ms; slope = 0.24, R2 = 1.00). Furthermore, the relationship between the measured susceptibility and concentration of hydroxyapatite also showed good linearity (TEs = 16.4, 20.8, and 25.2 ms; slope = − 0.00121, R2 = 1.00). In conclusion, the optimization of the TE in QSM makes it possible to obtain more detailed information regarding the susceptibility of biomaterials.






Similar content being viewed by others
References
Buch S, Liu S, Ye Y, Cheng YC, Neelavalli J, Haacke EM. Susceptibility mapping of air, bone, and calcium in the head. Magn Reson Med. 2015;73(6):2185–94.
Hopkins JA, Wehrli FW. Magnetic susceptibility measurement of insoluble solids by NMR: magnetic susceptibility of bone. Magn Reson Med. 1997;37(4):494–500.
Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004;52(3):612–8.
Wang Y, Liu T. Quantitative susceptibility mapping (QSM): decoding MRI data for a tissue magnetic biomarker. Magn Reson Med. 2015;73(1):82–101.
Wu Z, Mittal S, Kish K, Yu Y, Hu J, Haacke EM. Identification of calcification with MRI using susceptibility-weighted imaging: a case study. J Magn Reson Imaging. 2009;29(1):177–82.
Chen W, Zhu W, Kovanlikaya I, Kovanlikaya A, Liu T, Wang S, et al. Intracranial calcifications and hemorrhages: characterization with quantitative susceptibility mapping. Radiology. 2014;270(2):496–505.
Schweser F, Deistung A, Lehr BW, Reichenbach JR. Differentiation between diamagnetic and paramagnetic cerebral lesions based on magnetic susceptibility mapping. Med Phys. 2010;37(10):5165–78.
Wu B, Li W, Avram AV, Gho SM, Liu C. Fast and tissue-optimized mapping of magnetic susceptibility and T2* with multi-echo and multi-shot spirals. Neuroimage. 2012;59(1):297–305.
Hsieh CY, Cheng YC, Neelavalli J, Haacke EM, Stafford RJ. An improved method for susceptibility and radius quantification of cylindrical objects from MRI. Magn Reson Imaging. 2015;33(4):420–36.
Kressler B, de Rochefort L, Liu T, Spincemaille P, Jiang Q, Wang Y. Nonlinear regularization for per voxel estimation of magnetic susceptibility distributions from MRI field maps. IEEE Trans Med Imaging. 2010;29(2):273–81.
Wharton S, Schafer A, Bowtell R. Susceptibility mapping in the human brain using threshold-based k-space division. Magn Reson Med. 2010;63(5):1292–304.
Cronin MJ, Wang N, Decker KS, Wei H, Zhu WZ, Liu C. Exploring the origins of echo-time-dependent quantitative susceptibility mapping (QSM) measurements in healthy tissue and cerebral microbleeds. Neuroimage. 2017;149:98–113.
Sood S, Urriola J, Reutens D, O’Brien K, Bollmann S, Barth M, et al. Echo time-dependent quantitative susceptibility mapping contains information on tissue properties. Magn Reson Med. 2017;77(5):1946–58.
de Rochefort L, Brown R, Prince MR, Wang Y. Quantitative MR susceptibility mapping using piece-wise constant regularized inversion of the magnetic field. Magn Reson Med. 2008;60(4):1003–9.
Henkelman M, Kucharczyk W. Optimization of gradient-echo MR for calcium detection. AJNR Am J Neuroradiol. 1994;15(3):465–72.
Schofield MA, Zhu Y. Fast phase unwrapping algorithm for interferometric applications. Opt Lett. 2003;28(14):1194–6.
Zhou D, Liu T, Spincemaille P, Wang Y. Background field removal by solving the Laplacian boundary value problem. NMR Biomed. 2014;27(3):312–9.
Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage. 2012;59(3):2560–8.
Liu T, Xu W, Spincemaille P, Avestimehr AS, Wang Y. Accuracy of the morphology enabled dipole inversion (MEDI) algorithm for quantitative susceptibility mapping in MRI. IEEE Trans Med Imaging. 2012;31(3):816–24.
Gilbert G, Savard G, Bard C, Beaudoin G. Quantitative comparison between a multiecho sequence and a single-echo sequence for susceptibility-weighted phase imaging. Magn Reson Imaging. 2012;30(5):722–30.
Sun H, Kate M, Gioia LC, Emery DJ, Butcher K, Wilman AH. Quantitative susceptibility mapping using a superposed dipole inversion method: application to intracranial hemorrhage. Magn Reson Med. 2016;76(3):781–91.
Li J, Chang S, Liu T, Jiang H, Dong F, Pei M, et al. Phase-corrected bipolar gradients in multi-echo gradient-echo sequences for quantitative susceptibility mapping. MAGMA. 2015;28(4):347–55.
Wang Y. Quantitative susceptibility mapping: magnetic resonance imaging of tissue magnetism. Scotts Valley: CreateSpace Independent Publishing Platform; 2013. p. 57–82.
Blazejewska AI, Wharton S, Gowland PA, Bowtell R. Understanding the effects of oriented susceptibility inclusions on the phase and magnitude of gradient echo signals. In: Proceedings of 19th Annual Meeting ISMRM, Montréal, 2011; p 4511.
Robinson SD, Dymerska B, Bogner W, Barth M, Zaric O, Goluch S, et al. Combining phase images from array coils using a short echo time reference scan (COMPOSER). Magn Reson Med. 2017;77(1):318–27.
Shmueli K, de Zwart JA, van Gelderen P, Li TQ, Dodd SJ, Duyn JH. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med. 2009;62(6):1510–22.
Bilgic B, Fan AP, Polimeni JR, Cauley SF, Bianciardi M, Adalsteinsson E, et al. Fast quantitative susceptibility mapping with L1-regularization and automatic parameter selection. Magn Reson Med. 2014;72(5):1444–59.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kanazawa, Y., Matsumoto, Y., Harada, M. et al. Appropriate echo time selection for quantitative susceptibility mapping. Radiol Phys Technol 12, 185–193 (2019). https://doi.org/10.1007/s12194-019-00513-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12194-019-00513-x