Abstract
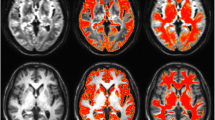
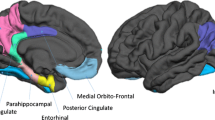
Our aim was to show whether sensitivity for detecting volume changes in regional gray matter in default mode network (DMN) at converted [from mild cognitive impairment to Alzheimer’s disease (from MCI to AD)] phase was improved by use of a standardized volume with global gray-matter volume. T1-weighted MR images (T1WI) of seven normal subjects and seven converted (from MCI to AD) patients were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. Gray-matter images segmented with Statistical Parametric Mapping 5 were measured by the atlas-based method. We focused on five nodes of the DMN. For each phase, region of interest (ROI) volumes in the five nodes were standardized by two methods: (1) the ratio to the screening phase (S_volume) and (2) the ratio to the screening phase after both volumes were standardized by the global gray-matter volume (S_N_volume). Significant group differences between longitudinal gray-matter volume change of the converted (from MCI to AD) group and that of the normal group were found in lateral temporal cortex by S_N_volume, and precuneus by S_N_volume. These findings are useful for improving the understanding of DMN volume changes at the converted (from MCI to AD) phase.


Similar content being viewed by others
References
Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and bold signals in resting-state functional connectivity maps. NMR Biomed. 1997;10:165–70.
Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8.
Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–51.
Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, et al. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17:766–77.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82.
Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95.
Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, et al. Top-down modulation of early sensory cortex. Cereb Cortex. 1997;7:193–206.
Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci USA. 2005;102:15700–5.
Fransson P. Spontaneous low-frequency bold signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29.
Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–42.
Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-pet studies in mci and ad. Eur J Nucl Med Mol Imaging. 2005;32:486–510.
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38.
Rombouts SA, Damoiseaux JS, Goekoop R, Barkhof F, Scheltens P, Smith SM, et al. Model-free group analysis shows altered bold fMRI networks in dementia. Hum Brain Mapp. 2009;30:256–66.
Wermke M, Sorg C, Wohlschlager AM, Drzezga A. A new integrative model of cerebral activation, deactivation and default mode function in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2008;35(Suppl 1):S12–24.
Fleisher AS, Sherzai A, Taylor C, Langbaum JB, Chen K, Buxton RB. Resting-state bold networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage. 2009;47:1678–90.
McCaffrey P, Fagan T, Landhuis E. Alzheimer research series on the default network. J Alzheimers Dis. 2010;19:747–58.
Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28.
Shin J, Kepe V, Small GW, Phelps ME, Barrio JR. Multimodal imaging of Alzheimer pathophysiology in the brain’s default mode network. Int: J Alzheimers Dis; 2011. p. 687945.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9.
Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–42.
Goto M, Abe O, Aoki S, Takao H, Hayashi N, Miyati T, et al. Database of normal Japanese gray matter volumes in the default mode network. J Magn Reson Imaging. 2014;39:132–42.
Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, et al. In vivo mapping of gray-matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14:298–309.
Matsuda H, Kitayama N, Ohnishi T, Asada T, Nakano S, Sakamoto S, et al. Longitudinal evaluation of both morphologic and functional changes in the same individuals with Alzheimer’s disease. J Nucl Med. 2002;43:304–11.
Busatto GF, Garrido GE, Almeida OP, Castro CC, Camargo CH, Cid CG, et al. A voxel-based morphometry study of temporal lobe gray-matter reductions in Alzheimer’s disease. Neurobiol Aging. 2003;24:221–31.
Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray-matter loss with voxel-based morphometry in mild cognitive impairment. NeuroReport. 2002;13:1939–43.
Frisoni GB, Testa C, Zorzan A, Sabattoli F, Beltramello A, Soininen H, et al. Detection of grey matter loss in mild Alzheimer’s disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002;73:657–64.
Fortea J, Sala-Llonch R, Bartres-Faz D, Bosch B, Llado A, Bargallo N, et al. Increased cortical thickness and caudate volume precede atrophy in psen1 mutation carriers. J Alzheimers Dis. 2010;22:909–22.
Fortea J, Sala-Llonch R, Bartres-Faz D, Llado A, Sole-Padulles C, Bosch B, et al. Cognitively preserved subjects with transitional cerebrospinal fluid SS-amyloid 1–42 values have thicker cortex in Alzheimer’s disease vulnerable areas. Biol Psychiatry. 2011;70:183–90.
Hyman BT, Gomez-Isla T. Alzheimer’s disease is a laminar, regional, and neural system specific disease, not a global brain disease. Neurobiol Aging. 1994;15:353–4.
De Lacoste MC, White CL 3rd. The role of cortical connectivity in Alzheimer’s disease pathogenesis: a review and model system. Neurobiol Aging. 1993;14:1–16.
Berg L, McKeel DW Jr, Miller JP, Storandt M, Rubin EH, Morris JC, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein e genotype. Arch Neurol. 1998;55:326–35.
Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–94.
Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–7.
Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Default mode network connectivity in stable versus progressive mild cognitive impairment. Neurology. 2011;76:511–7.
Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–44.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59.
Koch W, Teipel S, Mueller S, Buerger K, Bokde AL, Hampel H, et al. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? Neuroimage. 2010;51:280–7.
Johnson SC, Saykin AJ, Baxter LC, Flashman LA, Santulli RB, McAllister TW, et al. The relationship between fMRI activation and cerebral atrophy: comparison of normal aging and Alzheimer disease. Neuroimage. 2000;11:179–87.
Prvulovic D, Hubl D, Sack AT, Melillo L, Maurer K, Frolich L, et al. Functional imaging of visuospatial processing in Alzheimer’s disease. Neuroimage. 2002;17:1403–14.
He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, et al. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500.
Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–75.
Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–82.
Sharp DJ, Scott SK, Mehta MA, Wise RJ. The neural correlates of declining performance with age: evidence for age-related changes in cognitive control. Cereb Cortex. 2006;16:1739–49.
Otsuka Y, Osaka N, Morishita M, Kondo H, Osaka M. Decreased activation of anterior cingulate cortex in the working memory of the elderly. NeuroReport. 2006;17:1479–82.
Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–9.
Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the ‘‘Default network’’ In normal aging. Cereb Cortex. 2008;18:1856–64.
Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional–anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–62.
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94.
Marek T, Fafrowicz M, Golonka K, Mojsa-Kaja J, Oginska H, Tucholska K, et al. Diurnal patterns of activity of the orienting and executive attention neuronal networks in subjects performing a stroop-like task: a functional magnetic resonance imaging study. Chronobiol Int. 2010;27:945–58.
Shuter B, Yeh IB, Graham S, Au C, Wang SC. Reproducibility of brain tissue volumes in longitudinal studies: effects of changes in signal-to-noise ratio and scanner software. Neuroimage. 2008;41:371–9.
Ewers M, Teipel SJ, Dietrich O, Schonberg SO, Jessen F, Heun R, et al. Multicenter assessment of reliability of cranial MRI. Neurobiol Aging. 2006;27:1051–9.
Goto M, Abe O, Kabasawa H, Takao H, Miyati T, Hayashi N, et al. Effects of image distortion correction on voxel-based morphometry. Magn Reson Med Sci. 2012;11:27–34.
Goto M, Abe O, Miyati T, Kabasawa H, Takao H, Hayashi N, et al. Influence of signal intensity non-uniformity on brain volumetry using an atlas-based method. Korean J Radiol. 2012;13:391–402.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan.
Conflict of interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
For the Alzheimer’s Disease Neuroimaging Initiative.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of the ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
About this article
Cite this article
Goto, M., Abe, O., Aoki, S. et al. Longitudinal gray-matter volume change in the default-mode network: utility of volume standardized with global gray-matter volume for Alzheimer’s disease: a preliminary study. Radiol Phys Technol 8, 64–72 (2015). https://doi.org/10.1007/s12194-014-0295-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12194-014-0295-9