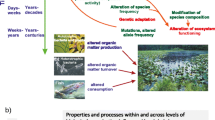
Abstract
The wild poses a multifaceted challenge to the maintenance of cellular function. Therefore, a multistressor approach is essential to predict the cellular mechanisms which promote homeostasis and underpin whole-organism tolerance. The intertidal zone is particularly dynamic, and thus, its inhabitants provide excellent models to assess mechanisms underpinning multistressor tolerance. Here, we critically review our current understanding of the regulation of the cellular stress response (CSR) under multiple abiotic stressors in intertidal organisms and consider to what extent a multistressor approach brings us closer to understanding responses in the wild. The function of the CSR has been well documented in laboratory and field exposures with a view to understanding single-stressor thermal effects. Multistressor studies still remain relatively limited in comparison but have applied three main approaches: (i) laboratory application of multiple stressors in isolation, (ii) multiple stressors applied in combination, and (iii) field-based correlation of multiple stressors against the CSR. The application of multiple stressors in isolation has allowed the identification of putative, shared stress pathways but overlooks non-additive stressor interactions on the CSR. Combined stressor studies are relatively limited in number but already highlight variable effects on the CSR dependent upon stressor type, timing, and magnitude. Field studies have allowed the identification of responsive components of the CSR to various stressors in situ but are correlative, not causative. A combined approach involving laboratory multistressor studies linking the CSR to whole-organism tolerance as well as field studies is required if we are to understand the role of the CSR in the natural environment.


Similar content being viewed by others
References
Barrett NJ, Thyrring J, Harper EM, Sejr MK, Sørensen JG, Peck LS, Clark MS (2022) Molecular responses to thermal and osmotic stress in arctic intertidal mussels (Mytilus edulis): the limits of resilience. Genes 13:155
Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR (2013) Genomic basis for coral resilience to climate change. Proc Natl Acad Sci 110:1387–1392
Bettencourt BR, Hogan CC, Nimali M, Drohan BW (2008) Inducible and constitutive heat shock gene expression responds to modification of Hsp70 copy number in Drosophila melanogaster but does not compensate for loss of thermotolerance in Hsp70 null flies. BMC Biol 6:5
Buckley BA, Owen M, Hofmann GE (2001) Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol 204:3571–3579
Bueno EM, McIlhenny CL, Chen YH (2023) Cross-protection interactions in insect pests: implications for pest management in a changing climate. Pest Manag Sci 79:9–20
Cheng J, Xun X, Kong Y, Wang S, Yang Z, Li Y, Kong D, Wang S, Zhang L, Hu X et al (2016) Hsp70 gene expansions in the scallop Patinopecten yessoensis and their expression regulation after exposure to the toxic dinoflagellate Alexandrium catenella. Fish Shellfish Immunol 58:266–273
Clark MS, Peck LS (2009) Triggers of the HSP70 stress response: environmental responses and laboratory manipulation in an Antarctic marine invertebrate (Nacella concinna). Cell Stress Chap 14:649–660
Clark MS, Geissler P, Waller C, Fraser KPP, Barnes DKA, Peck LS (2008) Low heat shock thresholds in wild Antarctic inter-tidal limpets (Nacella concinna). Cell Stress Chap 13:51–58
Clark MS, Sommer U, Sihra JK, Thorne MAS, Morley SA, King M, Viant MR, Peck LS (2017) Biodiversity in marine invertebrate responses to acute warming revealed by a comparative multi-omics approach. Glob Chang Biol 23:318–330
Clark MS, Thorne MAS, King M, Hipperson H, Hoffman JI, Peck LS (2018) Life in the intertidal: cellular responses, methylation and epigenetics. Funct Ecol 32:1982–1994
Clark MS, Peck LS, Thyrring J (2021) Resilience in Greenland intertidal Mytilus: the hidden stress defense. Sci Total Environ 767:144366
Collins M, Truebano M, Verberk WCEP, Spicer JI (2021a) Do aquatic ectotherms perform better under hypoxia after warm acclimation? J Exp Biol 224:jeb232512
Collins M, Clark MS, Spicer JI, Truebano M (2021b) Transcriptional frontloading contributes to cross-tolerance between stressors. Evol Appl 14:577–587
Collins M, Truebano M, Spicer JI (2022) Consequences of thermal plasticity for hypoxic performance in coastal amphipods. Mar Environ Res 177:105624
Côté IM, Darling ES, Brown CJ (2016) Interactions among ecosystem stressors and their importance in conservation. Proc R Soc B Biol Sci 283:20152592
Dong Y, Miller LP, Sanders JG, Somero GN (2008) Heat-shock protein 70 (Hsp70) expression in four limpets of the genus Lottia: interspecific variation in constitutive and inducible synthesis correlates with in situ exposure to heat stress. Biol Bull 215:173–181
Dong Y, Liao M, Han G, Somero GN (2022) An integrated, multi-level analysis of thermal effects on intertidal molluscs for understanding species distribution patterns. Biol Rev 97:554–581
Elowe C, Tomanek L (2021) Circadian and circatidal rhythms of protein abundance in the California mussel (Mytilus californianus). Mol Ecol 30:5151–5163
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Gracey AY, Chaney ML, Boomhower JP, Tyburczy WR, Connor K, Somero GN (2008) Rhythms of gene expression in a fluctuating intertidal environment. Curr Biol 18:1501–1507
Gunderson AR, Armstrong EJ, Stillman JH (2016) Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Ann Rev Mar Sci 8:357–378
Halpin PM, Sorte CJ, Hofmann GE, Menge BA (2002) Patterns of variation in levels of Hsp70 in natural rocky shore populations from microscales to mesoscales. Integr Comp Biol 42:815–824
Hamdoun AM, Cheney DP, Cherr GN (2003) Phenotypic plasticity of HSP70 and HSP70 gene expression in the Pacific oyster (Crassostrea gigas): implications for thermal limits and induction of thermal tolerance. Biol Bull 205:160–169
Hamer B, Hamer DP, Müller WEG, Batel R (2004) Stress-70 proteins in marine mussel Mytilus galloprovincialis as biomarkers of environmental pollution: a field study. Environ Int 30:873–882
Han Z, Truong QA, Park S, Breslow JL (2003) Two Hsp70 family members expressed in atherosclerotic lesions. Proc Natl Acad Sci USA 100:1256–1261
Hofmann GE, Somero GN (1996) Interspecific variation in thermal denaturation of proteins in the congeneric mussels Mytilus trossulus and M. galloprovincialis: evidence from the heat-shock response and protein ubiquitination. Mar Biol 126:65–75
Hu G, Tang J, Zhang B, Lin Y, Hanai JI, Galloway J, Bedell V, Bahary N, Han Z, Ramchandran R et al (2006) A novel endothelial-specific heat shock protein HspA12B is required in both zebrafish development and endothelial functions in vitro. J Cell Sci 119:4117–4126
Leeuwis RHJ, Gamperl AK (2022) Adaptations and plastic phenotypic responses of marine animals to the environmental challenges of the high intertidal zone. Oceanogr Mar Biol An Annu Rev 60:625–680
Lesser MP, Bailey MA, Merselis DG, Morrison JR (2010) Physiological response of the blue mussel Mytilus edulis to differences in food and temperature in the Gulf of Maine. Comp Biochem Physiol - A Mol Integr Physiol 156:541–551
Lindquist S (1986) The Heat-Shock Response. Ann Rev Biochem 55:1151–1191
Lockwood BL, Somero GN (2011) Transcriptomic responses to salinity stress in invasive and native blue mussels (genus Mytilus). Mol Ecol 20:517–529
Lockwood BL, Sanders JG, Somero GN (2010) Transcriptomic responses to heat stress in invasive and native blue mussels (genus Mytilus): molecular correlates of invasive success. J Exp Biol 213:3548–3558
Lockwood BL, Connor KM, Gracey AY (2015) The environmentally tuned transcriptomes of Mytilus mussels. J Exp Biol 218:1822–1833
Ramsøe A, Clark MS, Sleight VA (2020) Gene network analyses support subfunctionalization hypothesis for duplicated hsp70 genes in the Antarctic clam. Cell Stress Chap 25:1111–1116
Roberts DA, Hofmann GE, Somero GN (1997) Heat-shock protein expression in Mytilus californianus: acclimatization (seasonal and tidal-height comparisons) and acclimation effects. Biol Bull 192:309–320
Rodgers EM, Gomez Isaza DF (2021) Harnessing the potential of cross-protection stressor interactions for conservation: a review. Conserv Physiol 9:coab037
Rose NH, Seneca FO, Palumbi SR (2016) Gene networks in the wild: identifying transcriptional. Genome Biol Evol 8:243–252
Ruiz-Jones LJ, Palumbi SR (2017) Tidal heat pulses on a reef trigger a fine-tuned transcriptional response in corals to maintain homeostasis. Sci Adv 3:e1601298
Sagarin RD, Somero GN (2006) Complex patterns of expression of heat-shock protein 70 across the southern biogeographical ranges of the intertidal mussel Mytilus californianus and snail Nucella ostrina. J Biogeogr 33:622–630
Sinclair BJ, Ferguson LV, Salehipour-Shirazi G, Macmillan HA (2013) Cross-tolerance and cross-talk in the cold: relating low temperatures to desiccation and immune stress in insects. Integr Comp Biol 53:545–556
Smerdon GR, Chapple JP, Hawkins AJS (1995) The simultaneous immunological detection of four stress-70 protein isoforms in Mytilus edulis. Mar Environ Res 40:399–407
Spicer JI (2014) What can an ecophysiological approach tell us about the physiological responses of marine invertebrates to hypoxia? J Exp Biol 217:46–56
Takeuchi T, Koyanagi R, Gyoja F, Kanda M, Hisata K, Fujie M, Goto H, Yamasaki S, Nagai K, Morino Y et al (2016) Bivalve-specific gene expansion in the pearl oyster genome: implications of adaptation to a sessile lifestyle. Zool Lett 2:3
Todgham AE, Stillman JH (2013) Physiological responses to shifts in multiple environmental stressors: relevance in a changing world. Integr Comp Biol 53:539–544
Todgham AE, Schulte PM, Iwama GK (2005) Cross-tolerance in the tidepool sculpin: the role of heat shock proteins. Physiol Biochem Zool 78:133–144
Todgham AE, Iwama GK, Schulte PM (2006) Effects of the natural tidal cycle and artificial temperature cycling on Hsp levels in the tidepool sculpin Oligocottus maculosus. Physiol Biochem Zool 79:1033–1045
Tomanek L (2002) The heat-shock response: its variation, regulation and ecological importance in intertidal gastropods (genus Tegula). Integr Comp Biol 42:797–807
Tomanek L (2010) Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J Exp Biol 213:971–979
Tomanek L, Sanford E (2003) Heat-shock protein 70 (Hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (genus: Tegula). Biol Bull 205:276–284
Tomanek L, Somero GN (1999) Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J Exp Biol 202:2925–2936
Tomanek L, Somero GN (2000) Time course and magnitude of synthesis of heat-shock proteins in congeneric marine snails (genus Tegula) from different tidal heights. Physiol Biochem Zool 73:249–256
Uliano-Silva M, Americo JA, Brindeiro R, Dondero F, Prosdocimi F, Rebelo MDF (2014) Gene discovery through transcriptome sequencing for the invasive mussel Limnoperna fortunei. PLoS One 9:e102973
Wang J, Russell B, Ding MW, Dong YW (2018) Ocean acidification increases the sensitivity of and variability in physiological responses of an intertidal limpet to thermal stress. Biogeosciences 15:2803–2817
Yokoyama Y, Hashimoto H, Kubota S, Kuriyama A, Ogura Y, Mizuta S, Yoshinaka R, Toyohara H (2006) cDNA cloning of Japanese oyster stress protein homologous to the mammalian 78-kDa glucose regulated protein and its induction by heatshock. Fish Sci 72:402–409
Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Yang P, Zhang L, Wang X, Qi H et al (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490:49–54
Zhang G, Li L, Meng J, Qi H, Qu T, Xu F, Zhang L (2016) Molecular basis for adaptation of oysters to stressful marine intertidal environments. Annu Rev Anim Biosci 4:357–381
Funding
MC and MT are funded by the School of Biological and Marine Sciences, University of Plymouth. MSC is funded by NERC core funding to British Antarctic Survey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Collins, M., Clark, M.S. & Truebano, M. The environmental cellular stress response: the intertidal as a multistressor model. Cell Stress and Chaperones 28, 467–475 (2023). https://doi.org/10.1007/s12192-023-01348-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-023-01348-7